Showing 543 items matching "heat"
-
 National Wool Museum
National Wool MuseumTextile - Children's Jumper, Kathryn Knitwear, c.1964-1970
Kathryn Knitwear, founded by Robert Blake, manufactured high quality children’s knitwear in Melbourne from the 1940s – 1980s. Robert Blake began manufacturing children’s knitwear in his bedroom in Strathmore using a hand powered machine in the late 1940s. The operation moved to Ascot Vale and Essendon, before eventually establishing a factory in Moonee Ponds in the early 1950s. The business continued to expand, necessitating a move to a new factory in Broadmeadows. By 1962, the Broadmeadows factory was producing an average of 20,000 garments per month, which increased to 24,000 by 1964. Robert Blake’s Son, Brendan recalls that “The Kathryn brand was famous around Australia, anywhere children needed to keep warm and dress smartly. It also won a number of wool fashion awards”, including the 1969 Wool Awards, which was held by the Australian Wool Bureau and published in Women’s Weekly. The Kathryn range was designed for durability, comfort and care, without sacrificing style. They used patterning techniques that increase stretchiness, comfort and fit, as well as integrating decorative elements into the fabric to prevent them from being bulky, uncomfortable or tight. Making longevity of style a priority, Brendan Blake remembers that “there was one particular garment that was in the range for at least thirty years”. He also recalls “In the past, when women have found out that I was associated with Kathryn Knitwear, they would often relate to me the story of a garment they had purchased or received as a gift and, when their child had grown out of it, they would hand it on to another child. Several ladies have told me of purchasing garments for their daughters’ glory box, or saving a particular garment after their daughter had grown out of it. Brendan Blake: “At the peak of their operation they employed approximately two hundred people, mainly women and girls. A family would often seek to send their daughter to work in this company because they knew they would be looked after and safe. One lady wrote to me telling me that working at the Moonee Ponds Factory prior to getting married was the happiest time of her life.” In 1963, workers at the Kathryn factory earned £13 per week, which was 8 shillings and 8 pence higher than the minimum weekly wage for female workers in the textile manufacturing industry (£12 11s 4d). By 1970, the Kathryn Knitwear brand expanded from children’s knitwear into womenswear under the brand name ‘Lady Kathryn’, and for boys and men under ‘Robert Blake’. Continuing to diversify their distribution, they also began exporting ‘Kathryn’ garments to New Zealand, the Pacific Islands, and Japan. ‘Kathryn Knitwear’ was well-known for their early adoption of modern materials and techniques that had broad appeal to their customer base. This is shown in their early use of the acrylic fibre ‘Orlon’ in the 50s and 60s and ‘Superwash’ wool in the 1970s. Many of Kathryn Knitwear’s styles, particularly those that were long running staples of the brand, were available in both wool and Orlon to suit the consumer’s preference. Far from the humble origins of one man in his bedroom with a hand-cranked machine; at its closure in 1980, the Broadmeadows factory of ‘Kathryn’ housed more than 100 machines, including 53 sewing machines and 45 knitting machines. Robert Blake was “a passionate advocate for wool and Australian Made” throughout his whole life. A strong thread that ties through the lifespan of Robert Blake and Kathryn Knitwear is a balance between adopting new innovations without sacrificing the core values of durability, comfort, care and style that had made the brand so well known. Their legacy forms an integral part of both Australian social and manufacturing history.White short-sleeved jumper with all-over pattern of aqua blue diamonds. Blue floated threads slightly show through white on main body, leading to an overall pale blue effect on body, with white collar and cuffs. Closes with three pearlescent plastic buttons at back neck. .2 is a retail tag marked with the style code, and includes generic information on care for garments of different material composition..1) [label stitched into back neck of garment] KATHRYN REGD CREATED BY ROBERT BLAKE .2) [retail tag, intended to be folded in three, printed on both sides] [OBVERSE] KATHRYN Children’s Knitwear STYLE: [blue pen] S/35B SIZE: PRICE: / KATHRYN Garments are… • PRE-SHRUNK • STANDARD MEASUREMENTS • FIT EXACTLY • LAUNDER PERFECTLY / NOW .. KATHRYN GOES TO . . SCHOOL Ask your retailer for SCHOOL PULLOVERS by KATHRYN [REVERSE] WASHING INSTRUCTIONS WOOL Wash frequently to AVOID HEAVY SOILING Wash garment BY HAND, in lukewarm Velvet Soap suds. ON NO ACCOUNT RUB SOAP ON GARMENT. Squeeze suds gently through garment but DO NOT RUB. Rubbing will cause garment to thicken. RINSE AT LEAST TWICE IN CLEAN WATER TO REMOVE ALL SOAP. TO dry, roll garment in a towel to remove excess moisture, turn garment inside out and pull it lengthwise, DRY IN SHADE… AVOID SUNLIGHT. When dry, place brown paper or pressing cloth over garment and press with iron at correct heat. ORLON Wash as wool Lay flat to dry but DO NOT IRON. To keep brushed suits like new, brush frequently with nylon brush supplied. COTTON Wash by hand for preference in Velvet Soap suds. Rinse thoroughly in cold water and remove all excess water before drying on line. Please do not use any harsh detergent or bleach. Designed and manufactured by ROBERT BLAKE Pty. Ltd., MELB. (handwritten in pencil) S35 (untintelligible)/5 / NOW KATHRYN GOES TO SCHOOL Ask your retailer for SCHOOL PULLOVERS by KATHRYNknitwear, children's knitwear, clothing, children's clothing, jumper, manufacturing, fashion textile production, machine knitting, colourwork -
 Flagstaff Hill Maritime Museum and Village
Flagstaff Hill Maritime Museum and VillageCeramic - Stoneware Bottle, Henry Kennedy Pottery, Late 1800s to early 1900s
This bottle was made in Scotland and recovered decades later from a shipwreck along the coast of Victoria. It may have been amongst the ship's cargo, its provisions or amongst a passenger's personal luggage. It is now part of the John Chance collection. Stoneware bottles similar to this one were in common use during the mid-to-late 19th century. They were used to store and transport. The bottles were handmade using either a potter's wheel or in moulds such as a plaster mould, which gave the bottles uniformity in size and shape. The bottle would then be fired and glazed in a hot kiln. Makers often identified their bottles with the impression of a small symbol or adding a colour to the mouth. The manufacturer usually stamped their bottles with their name and logo, and sometimes a message that the bottle remained their property and should be returned to them. The bottles could then be cleaned and refilled. The Barrowfield pottery was founded in 1866 by Henry Kennedy, an Irish native, in the Camlachie district east of Glasgow, close to the Campbellfield and Mount Blue potteries. It is believed that Kennedy started with just one kiln but by 1871 was employing forty men and six boys and such was the success of the enterprise that by 1880, no less than eight kilns were in operation and a year later one hundred and the pottery was employing eighteen people. Stoneware bottle production was a mainstay of the pottery and over “1500 dozen” were being turned out daily along with other wares, including 30-gallon ironstone containers. With so many kilns in operation, six hundred saggars were required every week but, unlike some potteries, these were made on the premises from Garnkirk and Glenboig fire clays. Pottery production reaches a high scale which presented a high risk of fire and Barrowfield was no exception. In April 1884 heat from a kiln set fire to the roof resulting in significant structural damage, the loss of unfinished wares alone amounting to £10,000 a very substantial sum in 1884. The pottery recovered from this reverse but then Henry Kennedy died in July 1890. The terms of his will indicated that he and his sons John and Joseph were partners and this was reflected in a change of title in the 1891-92 Post Office Directory to Henry Kennedy & Sons. Despite the growth of the business there was still space enough, however, to allow china, earthenware and glass retailers Daniel and John McDougall to commence production of their Nautilus wares there in 1894, the success of which allowed them to soon move to permanent quarters at the empty Saracen Pottery, Possil. In around 1900 John Kennedy left to resurrect the liquidated Cleland Pottery and although Barrowfield remained listed as Henry Kennedy & Sons, brother Joseph was in control. In 1911 Henry Kennedy & Sons Ltd was formed, with two of the four directors being the Kennedy brothers. The pottery’s growth to this point was reflected in the eighteen kilns the largest pottery kilns then recorded in Scotland. However, the disruption of the First World War and the combined effects of subsequent economic depression, US prohibition, hygiene regulations and competition from alternative materials posed severe challenges for stoneware potteries in the post-war years as they competed with each other for diminishing markets. Competitors such as Eagle and Caledonian Potteries fell by the wayside and finally, Barrowfield closed in 1929. This stoneware bottle is historically significant for its manufacture and use in the late 19th to the early 20th century. The bottle is also significant as it was recovered by John Chance, a diver, from a wreck on the coast of Victoria in the 1960s-70s. Items that come from several wrecks along Victoria's coast have since been donated to the Flagstaff Hill Maritime Village’s museum collection by his family, illustrating this item’s level of historical value. Stoneware was produced at Barrowfield pottery for the domestic and export markets, with South America being a large market. Barrowfield stoneware can be found throughout the world. Its longevity and abundant production makes the subject item a significant addition to the Flagstaff Hill Maritime Museum collection.Bottle, salt glazed stoneware, beige, some discolouration above base. Chip on base and on neck. Inscriptions stamped near base.Makers lozenge stamped, H Kennedy Barrowfield Pottery GLASGOW at base.flagstaff hill, warrnambool, flagstaff hill maritime museum, maritime museum, shipwreck coast, flagstaff hill maritime village, great ocean road, john chance, h kennedy pottery, stoneware, ironstone, pottery, barrowfield glasgow -
 Coal Creek Community Park & Museum
Coal Creek Community Park & MuseumBox, pill, 1930-1940 ref: Museum of Applied Arts and Sciences
As per another example in better condition displayed on shelf above Drawers 1+2 in Chemist ' Beechams Pills as sold by the proprietors St.Helens, Lancashire England. Beechams Pills Ltd. Melbourne VIC'. Earliest mention in Victorian Newspapers TROVE : Argus (Melbourne, Vic. : 1848 - 1957), Friday 19 December 1884, page 7 'A WONDERFUL MEDICINE BEECHAMS PILLS Are admitted by thousands to be worth above a guinea a box for bilious and nervous disorders such as wind and pain in tho stomach, sick headache, giddiness, fulness and swelling after meals dizziness and drowsiness, cold chills, flushings of heat, loss of appetite, shortness of breath costiveness, scurvy, blotches on the skin, disturbed sleep, frightful dreams, and all nervous and trembling sensations, &c The first dose will give relief in 20 minutes This is no fiction, for they have done it in thousands of cases. Every sufferer Is earnestly invited to try one box of these pills, and they will be acknowledged to be WORTH A GUINEA A BOX. For females of all ages these pills are invaluable as a few doses of them carry off all humours and bring about all that is required No female should be with-out them There is no medicine to be found to equal Beecham's Pills for removing any obstruction or Irregularity of the system. If taken according to the directions given with each box they will soon restore females of all ages to sound and robust health For a weak stomach, impaired digestion, and all disorders of the liver they act like "Magic, and a few doses will be found to work wonders upon the most important organs of the human machine They strengthen tho whole muscular system, restore the long lost complexion bring back the keen edge of appetite, and arouse into action with the rosebud of health, the whole physical energy of the human frame These are ' facts ' admitted by thousands embracing all classes of society, and one of the best guarantees to the nervous and debilitated Is Beechams Pills have the largest sale of any patent medicine in the world Full directions are given with each box Sold by all druggists and patent medicine dealers throughout the colonies'. Most recent article in Victorian newspapers : TROVE : Wodonga and Towong Sentinel (Vic. : 1885 - 1954), Friday 24 December 1954, page 1. 'MUM KNOWS BEST SHE KEEPS THE FAMILY FIT WITH BEECHAM'S PILLS SAFE because Beecham's Pills contain no harmful habit-forming drugs-they are a purely vegetable laxative. Pills balanced formula gives natural laxative action without harsh purgative effects banishes constipation. MOTHERS know how to keep growing children in their teens fit and happy-with Beecham' s Pill, the family laxative. TAKE Beecham's Pills WORTH A GUINEA A BOX'. Relevant local newspaper article reference : TROVE : Gippsland Times (Vic. : 1861 - 1954), Thursday 29 October 1942, page 1 'ln times like these old friends are best You will not have to go far before finding a friend who can tell you by personal experience how gentle and reliable Beecham's Pills are--and how effectively they banish head aches. digestive upsets and liverish ness. Purely vegetable....1/-....2/...per box Worth a guinea a box' Cylindrical wooden box with the remains of an orange, red and white printed label on top, containing small orb shaped pills.Label on lid : Beecham's pills...............Beecham's Pills Ltd., Melbourne, Vic.laxitive, pills -
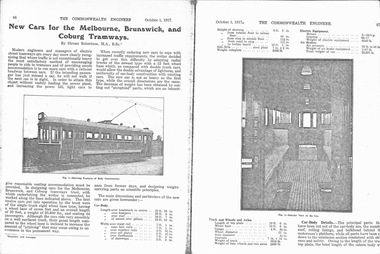 Melbourne Tram Museum
Melbourne Tram MuseumDocument - Report, The Commonwealth Engineer, Electrical Engineer, The Electrical Engineer and Merchandiser, Noiseless Tramcar - first Australian Vehicles - Bogies fitted have special noise reducing features, 1917 - 1951
Set of 12 reports, photocopied onto heat sensitive paper from various magazines. Documents match the image numbers. .1 - 2 pages, from The Commonwealth Engineer, 1/10/1917 - "New Cars for the Melbourne Brunswick and Coburg Tramways", written by Straun Robertson. .2 - 2 pages, from The Commonwealth Engineer, 1/3/1919 - "Double Bogie Combination Tram Car - St Kilda Brighton Electric Line". .3 - 2 pages - Electrical Engineer - 15/6/1924 - "One Man cars for Melbourne and Geelong Vic. The Brill Birney Safety Car" .4 - 1 page - Electrical Engineer - 15/11/1925 - "Standard Car for Melbourne Tramways" - has sketch of W2 369. .5 - 2 pages - Electrical Engineer - 15/8/1927 - "New Bogie car for Melbourne Tramways" Y class. .6 - 2 pages - Electrical Engineer -15/3/1936 - "Tramcar of New type for Melbourne - Large car for Two-man or One-man operation" - Y1 class .7 - 3 pages, The Electrical Engineer and Merchandiser - 15/3/1932 - "Modern Tramcars for Melbourne - Design for reduction of noise and construction with electrical Welding" W3 class. .8 - 2 pages, The Electrical Engineer and Merchandiser - 15/11/1933 - "New Tramcars for Melbourne" - has sketch of the W4 class tram. .9 - 3 pages, The Electrical Engineer and Merchandiser - 16/12/1935 - "Melbourne's Lates Tramcars, comfortable Accommodation and modern traction equipment" - W5 class .10 - 3 pages - The Electrical Engineer and Merchandiser - 15/3/1939 - "Improved Type Tramcar - advanced truck design, pneumatically operated doors, special lighting, acceleration 3pmh per sec." SW5 class. .11 - 3 pages - The Electrical Engineer and Merchandiser - 15/9/1950 - "Noiseless Tramcar - first Australian Vehicles - Bogies fitted have special noise reducing features - motor drive through bevel gears, dynamic braking" - PCC 980 (See also Reg Item 5601 for a similar report) .12 - 1 page - handwritten on the rear of a copy of item 11 - Editorial from the Oct. 1951 issue of same magazine looking at the rate of acceleration. Reprint of .7 added 30/7/2019, from papers ex Robert Green - in poor condition, has been folded, both left and right hand edges in multiple tears. The photos are good. Measures 282H x 220W.trams, tramways, mmtb, mbctt, new tramcars, vr, bogie trams, birney, x class, w2 class, y class, y1 class, w3 class, one man trams, w4 class, w5 class, sw5 class, pcc class, tramcar design, electrical engineering -
 University of Melbourne, Burnley Campus Archives
University of Melbourne, Burnley Campus ArchivesPhotograph - Colour slides, Thomas H. Kneen, Burnley Views, 1956-1969
Contributor: T.H. KneenBox of 47 slides, some labelled. (1) "Marjorie Hall 1st Year Student June '56 No. 1872." Working with fruit tree stock. (2) "Orchard June 1956 No. 1710." 2 men and a tractor.(3) "Camp 1957." Wilson's Promontory. (4) View of Drive No. 1677." C. 1958 (5) "GenView No. 1771." (6) "Burnley Gardens Entrance 4.4.58 N0. 1672." (7) "Wilson's Promontory Lilly Pilly Gully Nov 1960." (8) Dec '62." (9) "Dec '62." Kneen child. (10) Luffmann Ponds "Aut. 1962." (11) Grevillea Sep 1963. (12) Sep 1963.Reflection of Crack Willow in Luffmann Ponds. (13) Sep 1963. Orchard blossom. (14) View of Administration Building at sunset Aug 1964. (15) Rose 'Heat Wave.' May 1965. (16) Kneen child sitting under a tree May 1966. (17) 'Department of Agriculture Burnley Gardens' sign May 1966. (18) Administration Building May 1966. (19) Plant Science Block May 1966. ((20) Wintersweet August 1966.(Actually appears to be Witch Hazel Hamamelis mollis.) (21) Student on tractor in the Orchard October 1966. (22) Garden view, view of Principal's Residence through blossom trees October 1966. (23) Students walking through the Gardens October 1966. (24) "Leaf Cuttings Rex begonia 2 October 1966. (25) Emily Gibson beds October 1966. (26) Principal's Residence in a garden view October 1966. (27) Garden view looking towards the Principal's Residence and the Shady Garden October 1966. (28) Drive looking towards the Administration Building from the Plant Research Institute. (29) Kneen child (not Burnley?) (30) Unveiling of Burnley Horticultural College plaque commemorating 75 years, 1891-1966 - Eric Littlejohn, ?, T.H. Kneen. Includes key to Pavilion 1969. (31) "Rose Pruning Demo - Canteen." July 1969. (32, 33) "Rose Pruning Demo 1969." July 1969. (34) "Plant Science Block & College." July 1969. (35) Plant Science Block 6/69." July 1969. (36) "Burnley Gardens 6/69." looking towards Dairy and Yarra Boulevard (37) "Pond No. 1717." (38) "Pond 1748." Kneen children playing by the Luffmann Ponds, Oak tree behind.. (39) Ginkgo leaves June 1967. (42) ? (43) Erithyna caffra in flower (removed 2016) December 1966. ((44) Administration Building and Nursery from PRI." (45) Pelargoniums. (46) Azalea mollis (47) Pelargonium foreground, geranium background.marjorie hall, students, fruit trees, orchard, tractor, wilson's promontory, burnley gardens, entrance, drive, garden view, luffmann ponds, grevillea, rose, kneen family, sign, wintersweet, plant science block, administration building, students working outside, principals residence, plaque, rose pruning demonstrations, ginkgo, pelargoniums, erythryna -
 Flagstaff Hill Maritime Museum and Village
Flagstaff Hill Maritime Museum and VillageNail, circa 1810
This copper nail, sometimes known as a ‘Dumpy Bolt’ or spike, was salvaged from the hull of the wreck of the “George III”. It dates back to at least 1810. It was found by an abalone diver on the south east coast of Tasmania. The nail would have been used to hold the layers of the ship’s keel frame and the planking together. The nail has been passed from the abalone diver to an interested business man on a trip to the south of Hobart, on again to the business man’s close friend who then donated it to Flagstaff Hill Maritime Village. The metal of nails such as this one, after being in the sea for a long time, become affected by the natural reaction of the sea water, causing it to degenerate and thin, and the stress from the force of the sea over the years alters its shape. Iron nails had been used on ships previously, but they quickly corroded in the salt; ships needed regular, costly and time-consuming maintenance to replace the iron nails. Towards the end of the 18th century the British Navy trialled the use of copper nails, finding them to be very successful. Merchant ships began to adopt this process in the early 19th century, although it made ship building very expensive and was more often used for ships such as the “George III” that sailed on long voyages. The three masted sailing ship “George III” was a convict transport ship built in Deptford, England, in 1810. On 14th December 1834 she left Woolwich, England, bound for Hobart Town, Van Diemen’s Land (Tasmania), under Captain William Hall Moxey. She was carrying 220 male convicts plus crew, guards and their families, totalling 294 persons (another 2 were during the voyage). Amongst the cargo were military stores including several copper drums of gun powder. On 27th January 1835 the “George III” was near the Equator, about half way into her journey. A fire broke out and the gun powder was in danger of explosion, threatening the whole ship. Two convicts braved the heat and smoke, entered the store and seized the gun powder drums, suffering burns for their efforts but saving a probable disaster. The fire destroyed some of the provisions and food was scarce. Many became ill with scurvy and some died during the journey. Nearing the end of their journey on 10th April 1835 the “George III” was headed through the D'Entrecasteaux Channel, south east Tasmania, between the mainland and Bruny Island. She was sailing in the moonlit night to hasten her arrival in port due to the great number of sick on board. She struck uncharted rocks, known only to the local whalers, between Actaeon Reef and Southport Lagoon and within hours began to break up. The ship’s boats were used to first rescue the women and children. Firearms were used to help quell the panic of the convicts below decks and some were killed by the shots. Many convicts, including the sick, were drowned. In all, 133 lives were lost including 5 of the crew, guards and their families. It was the third worst shipping disaster in Tasmanian waters. A monument in honour of the prisoners who perished in the “George III” has been erected, noting the date of the wreck as “Friday 10th April 1835.” (NOTE: there are a few differences between sources regarding dates of the shipwreck, some saying March and others April 1835. There are also differences in the figures of those on board and the number of lives lost.) The copper nail is significant as an example of sailing ship construction; fasteners used in the early 19th century on ships carrying convicts to Australia. The nail is also significant for its association with the ship “George III”. The “George III” is registered on the Australian National Shipwreck Database, ID 7195 as an Historic Shipwreck. She is the third worst shipwreck in Tasmanian waters. She is also associated with Early Australian History and the transportation of convicts to Australia. The incident of the fire on board and the bravery of the convicts in making the gun powder safe is an example of the social character of the people in early Tasmanian colonisation. Copper nail (also called a Dumpy bolt or spike) from the convict ship George III, wrecked in 1835. Nail is long, bent in an ‘L’ shape about 3/5ths along, tapering from both ends to the bend. Both ends are flat and do not taper to a point, nor have a thread. The shorter end has been polished, showing bright copper. There is pitting along the nail and virdigris is evident on the longer, unpolished end. The nail is displayed with the longer section resting on a wooden board between two ‘U’ shaped uprights, the shorter section upright. flagstaff hill, warrnambool, shipwrecked coast, flagstaff hill maritime museum, maritime museum, shipwreck coast, flagstaff hill maritime village, great ocean road, george iii, ship construction, ship nail, 1835 shipwreck, 19th century shipwreck, william moxey, d'entrecasteaux channel, convict transportation, copper nail, dumpy bolt, spike, keel nail -
 Puffing Billy Railway
Puffing Billy RailwayEquipment - Victorian Railways Carriage Foot Warmer
During prestige, long distance train journeys some carriages had air-conditioning, and the majority of passengers had to brave unheated carriages. To offer some comfort during the winter months, the non-air-conditioned carriages were provided with footwarmers. These were metal containers roughly 100 mm thick and 300 mm wide, and about 750 mm long, which were filled with salt crystals (concentrated crystalline hydrated sodium acetate). The footwarmers were covered by sleeves of thick canvas, and two footwarmers were usually placed in each compartment of non-air-conditioned carriages. To activate the chemicals, the footwarmers were heated almost to boiling point. This was done by removing the canvas sleeves and placing the footwarmers in a large bath of very hot water. After they had been heated, they were removed from the bath and the sleeves refitted. They were then ready to be placed in the carriages. The McLaren patent foot warmer was used on railways in New South Wales, Queensland, Victoria and South Australia as well as South Africa and New Zealand. It was during the 1901 royal visit by the Duke and Duchess of Cornwall that these foot warmers were first used in New Zealand in the royal carriage. Before railway carriage heating was introduced, McLaren patent foot warmers were placed on the floor of New South Wales government railway carriages from 1891 to provide a little passenger comfort. The rectangular steel container worked a bit like a hot water bottle but instead of water contained six and a half kilograms of loosely-packed salt crystals, (concentrated crystalline hydrated sodium acetate). This was permanently sealed inside the container with a soldered cap. After the foot warmer was heated in vat of boiling water for about one and a quarter hours the crystals became a hot liquid. (The melting point for sodium acetate is 58 degrees). There was a whole infrastructure of special furnaces set up at stations for the daily heating of foot warmers. By 1914 the Victorian railways had 4,000 foot warmers in service and by 1935 there were 33 furnaces at principal stations to heat them. After about 10 hours the container was picked up by the handle and given a good vertical shake which helped the cooled liquid reform into a solid mass of hot crystals. Staff or sometimes passengers shook them en route when the foot warmers began to get cold. However, as they were heavy this was only possible by fit and agile passengers. At the end of the journey the containers were boiled again for reuse on the next trip. Sodium acetate railway foot warmers were introduced in Victoria in 1889, Adelaide to Melbourne express in 1899. "Shaking up" on this service took place at Murray Bridge and Stawell on the tip to Melbourne and at Ballarat and Serviceton on the trip to Adelaide. The use of foot warmers began to decline in New South Wales from the 1930s with the first trial of carriage air-conditioning in 1936, steam heating from 1948 ad LP gas heating from 1961. By the early 1960s the main services using foot warmers were the overnight mail trains. info from : http://www.powerhousemuseum.com/collection/database/?irn=67564#ixzz4UBNzVf6t Under Creative Commons License: Attribution Non-Commercial There was a whole infrastructure set up at stations for the daily heating of foot warmers in special furnaces. In Victoria alone in 1935 there were 33 heating works.Historic - Victorian Railways - Carriage Heater - Foot warmerA rectangular-shaped stainless steel casing with a welded seam down the back and welded ends. There is a handle at one end for carrying and shaking. Inside the foot warmer are two baffle plates and three trays to contain the sodium acetate. There was a cast-iron ball in each internal compartment. puffing billy, victorian railways, carriage haeter, foot warmer, passenger comfort, station furnace, railway ephemera, early heating methods -
 Surrey Hills Historical Society Collection
Surrey Hills Historical Society CollectionPhotograph, Mont Albert Central School Grade 1, 1921, 1921
Mont Albert State School was officially opened on 23rd April 1917. The school became Mont Albert Central School in 1918, taking in Forms 1 and 2. The school remained a Central School until 1964, when the secondary years formed the basis of a new High School, the Box Hill North High School, later to be named Koonung Secondary College. This is part of a large collection of material related to the Deakin, Mair and Young families, all with connections to Surrey Hills and Mont Albert. Ernest Lance Young was the son of Ernest Augustus Young (1891-1985) and Ruby Nichell Whitby (1892-1984). Lance was born 24 March 1915 in Surrey Hills. The family lived at 5 York Street, Surrey Hills. Electoral roll for 1937 gives the house name as 'Whitby Lodge'. He married Beryl Mair in 1939 and died on 5 October 1999 at Mont Albert. Electoral rolls list him as a manufacturer. His address after marriage was 11 York Street, Mont Albert. He is buried in Box Hill Cemetery (M-*-0867) along with his father. He served in WW2 (Service Number - VX104733 enlisting at St Kilda) and after returning took over his father's business. Young's Motor Products have manufactured products for the automotive and other related industries, including chemical trades, since 1920. Business history: Young's commenced trading in 1917 when Mr Ernest Augustus Young started selling paint brushes. At this time the company was known as E.A.Young & Co. with business premises in Queen Street Melbourne. Ernest soon expanded into paints and other products for the rapidly growing automotive trade and by 1920 was well recognised as a leading supplier. At this time canvas hoods were the norm and Ernest produced a "Canvas Hood Dressing" which gained acceptance as 'the one to buy'. This product was exported throughout the world. By 1930 Young's range had expanded and the product range included items like distilled water, gasket cement, vulcanising heat patches, rust prevention and many more diverse products. Young's name then, was so well known in Australia and the world, that a letter could be addressed just "Young's Melbourne" and it would reach the company. Young's survived the great depression, but in 1939 the Australian government commandeered the factory with all plant and equipment, thus closing Young's for the duration of the WW2. Ernest continued to make products at home for the war effort. When his son, Lance, returned home from overseas war service in Singapore, the Young's factory was re-established at 405 Canterbury Road, Canterbury near Chatham Station and worked to regain markets lost in the 1940s. By 1980 Lance Young wished to retire, his immediate family didn't want to continue the business and Lance believed Australia would lose a great asset if he just closed the company. He sought to find someone within the motor trade who would uphold the Young's principles of product and service and in 1981 Allan Kennedy & Sons bought the business.Lance Young was retained as an active consultant until his death in October 1999, aged 84. Products: Superseal for radiators, tyre dressing (tyre black), car shampoo, hood dressing, leather and vinyl cleaner. The factory was later elased to B&D Rollerdoors. REF: Personal communication (Laurie Newton, nee Young) and http://youngsmp.com.au/comprof.htm Black and white class photo taken outside the school building. The class of 18 girls and 22 boys is flanked by a male teacher of the LHS of the photo and a female teacher on the RHS. Children are wearing a variety of clothing indicating the absence of an official school uniform.REAR: Possibly 4 different hands as follows: 1. In black ink faded to brown: "January 1921 / Mont Albert State School" 2. "ERNEST" in black biro; looks to be a later insert to "Lance Young" in blue biro or ink. 3. "2nd on left / FRONT ROW legs crossed" in blue biro. -
 Flagstaff Hill Maritime Museum and Village
Flagstaff Hill Maritime Museum and VillageEquipment - Foundry Patterns set, Briggs Brass Foundry, Early 20th century
The wooden crate contains a set of patterns or moulds that were once used at Briggs’ Brass Foundry for making sand moulds. The traditional method of sand casting is over 2000 years old. It is part of a crafted process used to make brass and copper alloy goods suited to marine use; bells, boat hooks, cowls, propellers, handles, lids, rowlocks, hooks, letters, bolts, rail holders, brackets, deck plates, flanges, rudder guides, portholes and covers. Briggs’ Bronze is a copper-based alloy made from local ingots of copper, tin, zinc and lead in carefully measured quantities. The finished product is non-corrosive and can last indefinitely. The crate of patterns was donated by the Briggs family in the early years of Flagstaff Hill, along with other related items such as brassware, tools and machinery. The donated items were displayed in a simulated Brass Foundry in the Village. The items were on show from the completion of the building in 1986 until 1994 when the building was repurposed. The patterns represent the trades of foundering and metalwork, both supporting maritime industries such as shipwrights and boatbuilders. Farmers, manufacturers and other local industries also needed the castings made by foundries. The Brass Foundry display was one of the early ‘working craft’ shops at Flagstaff Hill. It included a historic Cornish chimney that was set up as a working model, telling the story of heat from furnaces to smelt metal, which would then be poured into the sand moulds. This chimney is made from specially curved bricks and is about two-thirds of its full height when originally located at the Grassmere Cheese factory. HISTORY of BRIGGS BRASS FOUNDRY: - The family business was founded in 1912 by Herbert Harrison Briggs (1963-1931) with his son George Edward Briggs, trading as Briggs & Son Foundry at 70 Wellington Street, Collingwood. Younger son Cyril Falkiner McKinnon Briggs joined the foundry in 1922, and it was renamed H H Briggs & Sons Foundry. Both sons ran the firm after Herbert’s death in 1931, making products mainly for marine purposes. They became Bell Founders in 1936 and were known for their specialty of high-quality ship bells. They produced miniature varieties of these and other decorative items such as small propellers. The firm became known as Briggs Marine Foundry. The great-granddaughter of Herbert Briggs inherited the Briggs Brass Bell, similar to the one at Flagstaff Hill. Cyril became the sole family member of the firm in 1965. The Briggs Marine was an exhibitor at the 1965 Boat Show, where he advertised as “non-ferrous founders” and “Bell Specialists”. The foundry relocated to Chesterville Rd, Moorabbin. Cyril passed away in 1967. It is thought that either Cyril or his business partner Frank Lee donated the objects from the Briggs’ Foundry around the time when the business moved to Moorabbin. However, Flagstaff Hill hadn’t been thought about until 1972. The donated items were registered in the Collection in 1986 but they could have been in storage from an earlier date. In October of that same year, Briggs Marine restored Schomberg Bell, a shipwreck artefact from the collection at Flagstaff Hill. Peter Oram, who had worked for the previous owners of Briggs Marine as a fitter and turner, took over the firm in 2014, reviving some of the old casts for current use. The business is now located at Seaford in Victoria and is part of Alliance Casting & Engineering Solutions (Alliance Casting Pty Ltd). In 2016 the original Collingwood Foundry building was repurposed as a thriving business hub named The Foundry. The crate and its patterns are significant for their association with brass foundries locally and generally in coastal areas of Victoria. Marine industries such as ship and boat building rely on good quality castings for their machinery, equipment and fittings. The patterns are associated with the long-running firm, Briggs Brass Foundry, that specialised in cast goods for the marine industry, ready to supply the needs for once-off or mass-produced items. Their products would have been fitted to sail and steam vessels along coastal Victoria including Warrnambool. Briggs Marine is also associated with the Schomberg Bell in Flagstaff Hill, restoring the bell to is former state to show an example of the bell from a luxury mid-19th century vessel. The craft of sand-casting from carved wooden patterns to create metal is an example of skills from the past that are still used today. Wooden rectangular crate with removable wooden lid. Inside is a set of wooden patterns of various shapes and sizes for making sand moulds in a metal foundry. The crate is made from thick wooden planks nailed together. The extended wooden struts on the long sides form a frame to hold the wooden lid. A pair of metal handles are at each short end of the crate, fixed with strong metal bolds. Between each pair of handles is an inscription stamped into the wood. The underside of the crate has red paint splashes. There are insect holes in the wood but no sign of current infestation. Stamped: "H.33 / II" (H may be N or a square B)flagstaff hill maritime museum and village, great ocean road, shipwreck coast, pattern, mould, foundry, brass foundry, metal foundry, crate, box, wooden container, briggs, traditional method, trade, sand cast, cast, brass alloy, copper alloy, marine equipment, marine tools, marine fittings, briggs' bronze, copper tin zinc lead, non-corrosive, briggs family, brassware, metalware, foundering, metalwork, maritime, casting, cornish chimney, curved bricks, grassmere cheese factory, 1912, herbert harrison briggs, h h briggs, george edward briggs, briggs & son foundry, collingwood, cyril falkiner mckinnon briggs, cyril briggs, h h briggs & sons foundry, bell founders, schomberg bell, alliance casting & engineering solutions, collingwood foundry, ship chandlers, marine products, flagstaff hill, warrnambool, maritime museum, maritime village, briggs & son brass foundry, briggs marine, moorabbin -
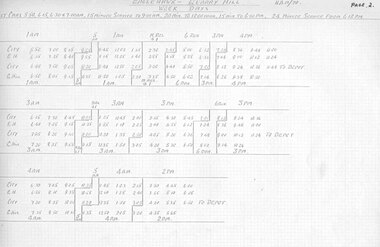 Ballarat Tramway Museum
Ballarat Tramway MuseumDocument - Roster, State Electricity Commission of Victoria (SECV), Rosters for Bendigo, 1970
Significant in being a almost complete set of rosters for Bendigo prior to closure - appear to be have been copied for a discussion or information document for management at Ballarat. Need to ascertain whether they line up with the actual last timetable. No Sunday tables given - probably did not change them.Set of photocopies of Rosters for Bendigo. Not sure whether these were actually used or were at the stage of a discussion document - see sheet 30. Photocopied onto foolscap size heat sensitive paper and some loosing information. Sheets have been numbered. Generally dated "HAM/70" Page 1 not location 2 - Eaglehawk - Quarry Hill Weekdays 3 - ditto - continued 4 - Golden Square - North Bendigo Weekdays - two different rosters given on two separate sheets. 5 - Eaglehawk - Quarry Hill Saturdays 6 - ditto continued 7 - Golden Square - North Bendigo - Saturdays 8 - Tables - 1AM, 2AM, 3AM 9 - Tables - 4AM, 5AM, 9AM - second sheet gives slightly different arrangements for 5AM 10 - Tables - 10AM, 11AM, 9Assist - second sheet gives slightly different arrangements for 10 & 11AM 11 - Tables - 2PM, 3PM, 4PM 12 - Tables - 6 run, Meal relief & 1, 11 Assist 13 - Tables - 9PM, 10PM, 16 Assist 14 - Tables - 1AM, 2AM, 3AM Conductors 15 - Tables - 4AM, 5AM, 6 run Conductors 16 - Tables - 2PM, 3PM, 4PM Conductors 17 - Tables - 12 Assist Motorman and 13 Assist Conductor - 2 sheets, can't see a difference. 18 - Tables - Saturday, 1AM, 2AM, 3AM 19 - Tables - Saturday, 4AM, 5AM, 9AM 20 - Tables - Saturday, 10AM, 11AM, 1st relief motorman 21 - Tables - Saturday, 9 Assist, 10 Assist, 14 Assist, 15 Assist 22 - Tables - Saturday, 1PM, 2PM, 3PM 23 - Tables - Saturday, 9PM, 10PM, Gol. Sq. relief Motorman 24 - Tables - Saturday, 1AM, 2AM, 3AM conductors 25 - Tables - Saturday, 4AM, 5AM, 1st Meal relief conductor 26 - Tables - Saturday, 1PM, 2PM, 3PM, conductors 27 - Tables - Saturday, 11 Assist, 12 Assist, 13 Assist - conductor 28 - Standby chart - Weekdays and Saturdays 29 - Rotation Roster for Motorman and Conductors - dated 12/5/1970 30 - Comparisons of Present Roster and proposed roster - includes cost impact analysis - 2 copies held. 31 - folded sheet - Weekday Instructions - poor order photocopied - 2nd copy - minor changes 32 - folded sheet - Saturday Instructions - poor order photocopied 33 - Daily hours of Motorman conductors - Weekdays and Saturday 33A - Hours of Motorman and Conductors - Sunday 34 - Weekly Hours - for motorman and conductors - poor order photocopied and data filled in on photocopy. 39 - Tramways - Motorman and Conductors Rotation Roster - dated 5-3-1970trams, tramways, rosters, timetables, sec, bendigo -
 Eltham District Historical Society Inc
Eltham District Historical Society IncMagazine, Sun News-Pictorial, Bush Fires: A pictorial survey of Victoria's most tragic week, January 8-15, 1939, 1939
THE WEEK REVIEWED (Article; Bush Fires: A pictorial survey of Victoria's most tragic week, January 8-15, 1939. Published in aid of the Bush Fire Relief Fund by the Sun News-Pictorial in co-operation with its newsagents, pp2-3) THE fiercest bush fires Australia has known since its discovery are quiescent at the moment, and Victoria, in the comparative coolness of the change which came with rain on Sunday night, has begun·to count its losses. In the fiery eight days, from Sunday to Sunday, at least sixty-six men, women and children have lost their lives in forest fires, or have succumbed to burns and shock; many others have died from heat; and several serious cases of burns are being treated in hospitals. Two babies in Narrandera district have died, and ten others are in hospital, because of milk soured by the record temperatures of those eight days. Forest damage totals at least a million pounds, and incalculable damage has been done to the seedlings which were to have been the forests of the future. Water conservation will be seriously affected by the silting-up of reservoirs and streams from which protective timber has been taken by the all-engulfing flames. More than a thousand houses have been destroyed, and these, with 40 mills, and schools, post-offices, churches, and other buildings, represent a loss of at least half a million. At least 1500 are homeless. For their aid, money raised in appeals has now passed the £50,000 mark, and the biggest relief organisation ever set up in peace time has swung into operation. The First Hint Victoria's first hint of what was to come appeared on Sunday, January 8, when most parts of the State awoke to find a blistering day awaiting. At 12.20 p.m., when the thermometer reached its highest for the day, 109.6 degrees, the first fire victims were at that moment going to their death on a bush track five feet wide off the main road to Narbethong. They were the forestry officers Charles Isaac Demby and John Hartley Barling, who went to warn Demby of his danger when he parted from his companions, and was himself surrounded by the treacherous fire. It was not until 8 o'clock next morning that the tragic news was flashed throughout the State. Searchers found the two charred bodies close together, one seeking protection in the nook of two logs. Barling's watch had stopped at 1.20. In the meantime, tragedy was spreading its cloak. By Monday, big fires were raging at Toolangi, Erica, Yallourn, Monbulk, Frankston, Dromana, Drouin South, Glenburn, and Blackwood, with smaller outbreaks at many other centres. In the ensuing week, while women and children were evacuated as fast as the flames would permit, Erica-scene of the 1926 fire disaster-thrice escaped doom by a change of wind. Indeed, those who have been in the fire country these past days say that the numbers of times a change of wind has saved towns from destruction is amazing. In the towns they speak of miracles. Monday's Miracles The escapes from Monett's Mill at Erica and from the Hardwood Company's Mill at Murrindindi, near where Demby and Barling went to their death, were Monday's miracles. Twenty came out alive from each mill. At the first a 60ft. dugout provided an oven-like refuge; at the second, 12 women and children survived in the smoke-filled gloom of a three-roomed cottage while their eight men, their clothes sometimes afire, poured water on the wooden walls. Three houses out of ten remained when the fire had passed. Record Temperatures Sunday had been the hottest Melbourne day for 33 years; Monday dropped to a 76.1 degree maximum; but Tuesday dawned hotter than ever, the mercury reaching 112.5. By now rumor was racing ahead of fact; whole towns were being reported lost; the alarm was raised for scores of missing persons. But fact soon overtook rumor, and within a few days the staggering toll began to mount to a figure beyond the wildest imaginings of the panic-stricken. Six died from heat on this torrid Tuesday, and the fires spread in a wide swathe from south-west to north-east across the State. Fish died in shallow streams. A curtain of smoke hid the sky from all Victoria, and hung far out to sea. It alarmed passengers on ships. On the Ormonde, on the voyage to Sydney from Burnie, women ran on deck, believing fire had broken out in the hold. Days later the smoke reached New Zealand. In Melbourne thousands of fire-volunteers were leaving in cars: vans, motor-buses-anything reliable on wheels-to aid the country in its grim fight. In the fires at Rubicon and. Narbethong, seventeen were facing death this day. But not till Wednesday, when Melbourne breathed again in a cool change, while the country still sweltered in temperatures up to 117 degrees, did the news come through the tree blocked roads. A woman and her little daughter, trapped on the road, were among those who died. Their bodies, and those of menfolk with them, were found strewn out at intervals along the road, where the furnace of the surrounding fire had dropped them in their tracks as they ran. Twelve died at a Rubicon mill, five on the road at Narbethong. At Alexandra, not far distant, a baby was born while the fires raged, and stretcher-bearers brought in the injured. On Thursday the State Government voted £5000 for the relief of fire victims. The Governor (Lord Huntingfield) and the Lord Mayor (Cr. Coles) visited some of the stricken areas, and dipped into their pockets personally. Later, the City Council, too, voted £5000. Friday, The 13th Friday, the Thirteenth, justified its evil name. A blistering northerly came early in the morning, presaging destruction, and forcing the mercury to a new record of 114 degrees. Racing fires killed at least ten in those terrible 12 hours. Four children were engulfed in the furnace at Colac. Panic drove them, uncontrollable, into the smoke-filled road when the fire raced down behind their home. They choked to death. In other parts fires were joining to make fronts of scores of miles. Kinglake was being menaced on two fronts, £60,000 worth of timber was going up in smoke in Ballarat district. Warburton was surrounded. Residents at Lorne, favoured resort, were being driven to the sea-front by a fire which destroyed at least 20 homes. Healewille. with flames visible from the town at one stage, was in a trough between two fires which burned four guest-houses, seven homes and left its surrounding beauty-spots wastes of bowed-over, blackened tree-fern fronds; with its famous Sanctuary, however, intact. Most of Omeo was destroyed this black day: Noojee. while 200 residents crouched in the river, was being reduced to a waste of buckled iron and smoking timber; Erica was once again saved by a change of wind. Beneath a pall of smoke, the Rubicon victims were buried at Alexandra. Friday night and the early hours of Saturday saw the streets of beleagured towns strewn with exhausted fire-fighters. Their flails beside them, ready for the next call, they lay where exhaustion overtook them-on footpaths, beside lamp-posts, in gutters, in cars, under trucks. Saturday's dawn brought clear skies and lower temperatures in many parts, and from the burnt-out areas came a great rush of tragic reports. The death-roll rushed past the fifty mark with incredible speed. Some had been trapped on roads, others at mills; some, after burying their treasures, had clung too long to the places they had made their homes for many years. Four men lost their lives because one went back for his dog. By Sunday, when the first of the saving rain came, nearly another score of names had been added to the list.Newspaper magazine, 48 pages (incl. covers). Fully digitised and searchable PDFPublished in aid of the Bush Fire Relief Fund by the Sun News-Pictorial in co-operation with its newsagents.bushfires, 1939 bushfires, black friday, warrandyte -
 Eltham District Historical Society Inc
Eltham District Historical Society IncDocument - Property Binder, 1184 Main Road, Eltham
Newspaper article: A sustainable award, Diamond Valley Leader, 1 November2006, Architect and building Llewellyn Pritchard won resource Efficiency Housing Award, finalist in HIA Greensmart Building of the Year Award. House – Environmental Leader (Published: Nillumbik Now and Then / Marguerite Marshall 2008; photographs Alan King with Marguerite Marshall.; p186) In 2006 environmental awareness was mushrooming in the community, which is reflected in the award-winning house at Main Road near Wattletree Road, Eltham. At first sight, the building appears a mix of a classic Eltham mud-brick house and an avant-garde building style. The crown of solar panels stretching along the width of the curved roof, indicates that this is no ordinary house. In fact it signals a new building trend of minimal impact on the environment. Yet it utilises the environment with high technical expertise to achieve comfort and cut running and maintenance costs. In recognition of this, its designer/builder, Conscious Homes, won the 2006 National HIA Greensmart Resource Efficiency Award. For Conscious Homes director, Llewellyn Pritchard, this house reflects a philosophy, strengthened by his connection with Aboriginal culture, through his foster siblings. Pritchard believes the sustainable way indigenous Australians lived and their spiritual connection with land, demonstrates how humanity is part of the ecology. His interest in environmental design stemmed from growing up in bushy Eltham Shire, with its mud-brick tradition. This was followed by studying Architecture at RMIT in the early 1980s, and learning about passive solar design. Pritchard says this house demonstrates that environmental sustainability is not about sacrifice, but about exceptional levels of occupant comfort, savings in running costs and modern fittings and appliances.1 The solar panels on the north roofs are intentionally obvious to make a statement about what the building is doing. But inside the systems are hidden and interactive with conventional services, such as the underground water tank. The house is water and energy self-sufficient and at 12 squares is much smaller than conventional houses, to minimise resources. Yet it accommodates his family of four with three bedrooms, a living/dining and kitchen area and a bathroom/laundry. Importantly the building is designed to last hundreds of years, by being able to be modified as the need arises, such as for commercial use. In this way the structure minimises its environmental impact. The solid double mud-brick walls (which are insulated) include steel beams and supporting frame, allowing the future removal or alteration of any section. The materials are local, recycled and of low toxicity where possible.2 Inside and out, the mud-brick is rendered and sealed with a combination of cement and sand and a mud-based coating in a soft golden hue increases its life. Inside, the golden-brown timber is plantation Mountain Ash and the concrete floors throughout – of local stone aggregate with a clear seal – have a natural looking random stone appearance. The house sustains a stable temperature of around 20 degrees, assisted by the concrete slab floor. The many large double-glazed windows and highlights (windows set high on walls) provide cross-flow ventilation. The north-facing living area maximises heating from the lower winter sun and is cooler in summer, because the sun is higher. Heating comes from a solar hydronic slab system. All appliances and fittings are high efficiency energy or water rated. Appliances in the timber kitchen include a gas stove and a dishwasher, using the building’s own power and water. French doors open from the living area to a deck, concealing the treatment system for all waste water. This is pumped through sub-soil drippers to the indigenous garden beds and no-dig vegetable patch. Below the carport is the 80,000-litre rainwater tank and at the back, the boiler room houses the solar boiler, water tank access, domestic water supply pump, filter gear and hydronic slab heating controls. The solar system is backed up with gas, which is needed to heat water only in winter. Gas used is less than one quarter of that for an average home with ducted heating. Excess power is fed back to the grid and the building uses about one quarter of the mains electricity of an average home. Other local builders have followed Pritchard’s lead in resource efficiency for minimal environmental impact.main road, eltham, businesses, llewellyn pritchard, hia greensmart building of the year award., efficiency housing award, conscious homes australia pty ltd -
 Ballarat Tramway Museum
Ballarat Tramway MuseumDocument - Instruction Book, Westinghouse Brake Company of Australasia Limited and The Westinghouse Brake & Saxby Signal Co. Ltd. of 82 York Road and Kings Cross London, "Westinghouse Railway Operating Data", 2000
Photocopy of 54 data sheets published by Westinghouse Electric & Manufacturing Company of East Pittsburgh Pa, USA c1920. Consists of plastic cover, header page with Westinghouse logo, contents sheets (2 pages), forward, 67 pages (single side photocopy) and heavy rear card cover bound with a green comb binder. Original material lent by Craig Tooke of the Melbourne Tramcar Preservation Association at Haddon. Photocopied by Warren Doubleday March 2000. List of contents produced 30/6/2000 and then bound. Contains data sheets regarding motors, commutators, brushes, armatures, bearings, field coils, pinions, lubrication, air piping, axle collars, resistance grids, gear cases and other technical information. Westinghouse Railway Operating Data 30/6/2000 List of Contents Page No. Care and repair of commutators 1 Undercutting commutators 2 Railway Motor carbon brushes 3 Brush holders 4 Flashing of railway motors 5 Soldering railway armatures 6 Armature Winding 7 Banding armatures 8 Railway Motor Bearings 9 Lubrication of railway motor bearings 10 How to babbitt motor bearings 11 Oil, grease and waster for motors and gears 12 Saturation of motor bearing waste 13 Testing Polarity of Field Coils 14 Charging of storage batteries on Interurban & street rail cars 15 Precautions to be taken with blower installations on motor cars 16 Putting on Railway Motor Pinions 17 How to take armatures out of box frame motors 18 Dipping and Baking of Railway Motors 19 War time dipping and baking outfits 20 Dipping and baking railway motors will decrease troubles 21 Protection of Motor Bearings from Dust 25 Winter Operation of Railway Motor equipments 26 Installation of Air piping to prevent freezing 27 Maintenance of Traction Brake Equipment 28 Maintenance of controller fingers and contacts 29 Hand operated circuit breakers 30 Railway Motor Testing I 31 Railway Motor Testing II 33 Railway Motor Testing III 35 Railway Motor Testing IV 36 Railway Motor Testing V 37 Removing and replacing railway motor armature shaft 39 Mounting and Maintenance of car resistors 40 Lubrication of control apparatus 41 Maintenance of fuse boxes for railway service 42 Does it pay to dip and bake armatures 43 Dipping and Baking as a financial asset 44 Shop Organisation 45 Tinning Malleable Iron Bearing shells 46 Life of armature bearings or railway motors 47 The assembly of complete sets of commutator segments 48 Electric welding as a factor in reclamation 50 Metal to Metal press, shrink and clamping fit allowances 52 Life of railway motor carbon brushes 54 General information of grid resistance design for the operating man 56 Stopping a car by braking with the motors 57 Railway Motor shafts and their maintenance 58 Axle collars 59 Gear cases 60 Ventilated railway motors 62 Revamping Loose armature bearings 64 Life of axle bearings of railway motors 65 Heat-treated bolts for railway service 66 Document imaged over 7 parts 7-9-2016 - see hi res files. trams, tramways, westinghouse, motors, data sheets, technical information -
 Flagstaff Hill Maritime Museum and Village
Flagstaff Hill Maritime Museum and VillageEquipment - First Aid Case, Thomas Urquhart & Son Pty Ltd (Thos. Urquhart), 1930-1939
This small, portable 1930s Sanax First Aid Case has been strongly constructed, with corners reinforced with metal to take knocks and bumps, so it could be quickly transported to the site of an emergency. Having these supplies organised into a kit made them easily accessible and reduces time to take them to the site of the accident. It was possibly designed for use in factories because the booklet in the case states that the kit complies with “Part 1, Victorian Factories Regulations”. The text of the printed brand “Sanax First Aid Case” is right-way up when the case stands vertically on its hinged side. In modern times people are well aware of the importance of quick treatment when accident and injury occur. However, before the first commercial First Aid Kit was made by Johnson & Johnson in 1888, people had little knowledge about treating injuries and lacked information about suitable supplies to keep on hand for emergencies. They were often unaware of how to help in that critical time before the doctor or other assistance arrived, a particularly important time for the many people living in remote areas. A quote from Johnson’s & Johnson’s 1888 price list explains “It is a fact, which is everywhere being recognized, that many lives are lost and much suffering entailed in such accidents on account of the lack of the simple but necessary articles required to afford prompt assistance to the wounded.” One example of the value of First Aid assistance to community groups is shown in an article from the Weekly Times, 29th November 1930. It records a report from the Annuello Branch of the Younger Set (a Country Women’s Organisation), telling that on Armistice Day their president Mrs Jamieson, presented the Annuello School with the gift of a Sanax Red Cross First Aid outfit, which was accepted as being “of great practical use to the scholars.” (Annuello is a remote wheat growing area in the Mallee region of North Western Victoria, which became a soldier settlement area after World War I. There is a strain of wheat named ‘Annuello’ due to its suitability for that area. ) The Sanax Case in our Collection contains instructions, equipment and medical items suitable for use in emergency situations. The Case was one of 42 patterns available from Sanax that conformed to ‘Part 1, Victorian Factories Regulations’. It includes items made by Sanax Company and by Burroughs Wellcome & Co. (Australia) Ltd., Sydney, NSW. A quote at the back of the First Aid Emergency Instructions booklet says: “Sanax products are made in Australia by or under the supervision of qualified chemists, from the highest quality materials. They are dependable for the purposes written on labels.” BOOKLET included in First Aid Case: “SANAX” First-Aid Emergency Instructions has orange cover and white pages, joined in the centre by two staples. Booklet contains First Aid Instructions for general events listed in alphabetical order. It also contains an indexed sections headed “Poisoning, and what to do” written by S.A. Burrows, Ph.C., Vuc and N.Z. There are instructions and diagrams on how to perform Artificial Respiration. There are advertisement for Sanax products throughout the booklet that include; - Sanax Ambulance Stretcher for timber mills, mines, ships and quarries - Saw dust masks (porous rubber) for workers in dust, paint or duco sprayers Inside cover lists Sanax’s Australian made products including - tablets and powders for headaches, neuralgia, influenza, colds - snuff for Catarrh that is “quite harmless” - First Aid Cases that come in a range of 42 patterns - sunburn preventatives and treatments - healing salve for carbuncles, pock, pimples, boils, varicose ulcers etc. - snake bite outfits and kits LEAFLETS included in First Aid Case: (1) Tannafax Tannic Acid Jelly. Tannafax should be kept at hand in every home. It should be applied direct from the tube and used with neither oil nor grease. Where a large area has to be covered the clamped end may be torn or cut off to give a wider mouth to the tube. Collapsible tubes of different sizes. Made in Australia. Burroughs Wellcome & Co. (Australia) Ltd. (Incorporated in England). Sydney, NSW. Assorted Houses, London, New York, Montreal, Cape Town, Milan, Bombay, Shanghai, Buenos Aires. Copyright A. 1817, J. 9463 (2) Tabloid. The strong thing is the just - - . Tabloid marks the wor - - Burroughs Wellcome & Comp. The use of the word is to enab – the prescriber, dispenser and patient to get the right thing with one short word, instead of the firm’s long name. If another maker apply the word to his product, the act is unlawful. Tabloid is our trade mark and brand. If a vendor disregard it in dispensing or selling, the act is unlawful for the same reason. We prosecute both offenders rigorously, in the interest of prescribers, dispensers, patients and the owners of the trade mark. Please inform us of any instance of either offence. Burroughs Wellcome & Co. (Australia) Ltd. (Incorporated in England). Telephone Number - M 4184 (4 lines) All communications to G.P.O. Box No. 1185 DD. Copyright Sy. 20. & J 9894. Medicines and Equipment included in First Aid Case: - Absorbent Cotton, Sanax, for absorbing blood or drying a wound. As a swab for washing wounds; to place above a compress to keep the heat in: or as a pad to protect wounds or fractures. The Sanax Co. Manuf. Chemists, Melbourne. Regd. Office: 5 Brunswick St, Fitzroy. N.6. - ACHE tablets, Sanax, for all aches, pains, fevers etc. Dose: 2 to 3 tablets with a draught of water, every 3 hours. Children in proportion. For influenza or colds, take the bedtime dose with a hot lemon drink or toddy. Recommended for Headaches, Colds, Influenza, Fevers, Neuralgia, Rheumatism, Nerve Pains, Sleeplessness, and Seasickness. Three Sanax Ache tablets equals one Sanax Ache powder. Each tablet contains 1.75grs. each Phenacotinum and Acety acSzilcyl, and .75grs Ammon Brom. Etc.. Sanax brand specialties are prepared by highly qualified pharmaceutical chemists and may be accepted as safe and effective for the purpose indicated on the label. The Sanax Co. Melbourne - Eye lotion, Sanax, “in eye bath full strength or diluted with equal parts of water. Sanax Co. Brunswich St, Fitzroy, Melbourne. - Iodine, Sanax, POISON, with instructions for what to do if swallowed. - Kuraburn, Sanax, Applied to the burn and allowed to dry, the pain and heat instantly disappear, and blistering is prevented. If necessary, apply again in an hours. To safeguard against burning when sunbathing, apply before exposure to the sun. If already sunburnet, use Kuraburn as directions above. Safe and harmless. Sole makers, The Sanax Co. Brunswick St. - - Vic. - Sal Volatile, Sanax, - - stimulant for - - nervous aches - - or as smelling salts Dose - - - - Solution of A- - - 5%, . The Sanax Co. Brunswick St, Melbourne. - Tannafax, Burroughs Wellcome & Co. Australia Ltd. Sydney, N.S.W., 20gm. Approx., Tannic Acid Jelly, (Tannic Acid with 0.5% Phenol in a water-soluble base) for burns and scalds. A.N. 15050, p188, logo of a unicorn. Apply lightly, allow to dry, and bandage loosely. Do not apply oil or grease. - bottle wrapped in brown paper, unknown contents, paper adhered to bottle. - dish, kidney shaped, metal, white enamel with black rim - eye bath, green, plastic or Bakelite SANAX COMPANY The Sanax Company was at the address of 5 Brunswick Street, Fitzroy [Melbourne] at least as early as November 1924, as shown by its advertisement of Ache Powder in the Weekly Times, 8th November 1924. It was still at this address in September 1951, when it advertised First Aid outfits and components in the Post Master General’s section of the Commonwealth of Australia Gazette. REFERENCES: Annuello, Victoria; Wikipedia, https://en.wikipedia.org/wiki/Annuello,_Victoria Annuello Younger Set, Branch Activities and Local Reports, Country Women’s Organisations, Weekly Times, 29 November 1930, Trove http://trove.nla.gov.au/newspaper/article/224921009?searchTerm=%22sanax%22%20and%20%22melbourne%22&searchLimits=# Commonwealth of Australia Gazette, Issue 32, 24th April 1915, https://www.legislation.gov.au/file/1915GN32 [Johnson & Johnson Price List, September 1, 1888, p. 20. From our archives], Celebrating the 125th Birthday of the First Aid Kit , The Story of Johnson & Johnson, , http://www.kilmerhouse.com/2013/06/from-1888-to-2013-celebrating-the-125th-birthday-of-the-first-aid-kit/ Post Master General’s section of the Commonwealth of Australia Gazette, Issue No. 73, Thursday 27th September 1951 http://trove.nla.gov.au/newspaper/article/232185299?searchTerm=%22sanax%22%20and%20%22fitzroy%22&searchLimits= Sanax First Aid Emergency Instructions, by S.A. Burrows, publisher Sanax Ltd. Fitzroy, Victoria, 1930-1939 English, book, Illustrated edition, Trove http://trove.nla.gov.au/version/40948895 Access to emergency medical help in early settlement days of Victoria could take quite some time, especially in remote areas. From 1888 First Aid Kits and instructions became available for work sites, offices, community groups and individuals, helping to bridge the gap between the accident and the arrival of medical assistance. This portable Sanax First Aid Case is an example of portable medical equipment made in Melbourne, Australia, in the 1930’s and available to the public. It contains a range of items plus information to be used in a variety of injuries and emergencies in in factories, households, businesses and local communities, and instructions on their use. First Aid Case, portable, Sanax First Aid Case. First Aid kit in strong black cardboard carry case with metal reinforced corners, metal hinges on lid, metal catch and leather carry handle. Inside lid is a vertical strap with narrow gap behind it. Base is divided into two compartments. Manufactured by Sanax, Fitzroy, Melbourne, C. 1930-1939 Contents include "Sanax" First Aid instructions booklet, 2 leaflets, metal kidney dish enamelled in white with black trim on edge, green plastic or Bakelite eye bath, eye lotion, Tannafax tannic acid jelly, Sal Volitile, Kuraburn, Iodine, Argyrol, ACHE tablets, absorbent cotton in cardboard box, gauze bandage, and UNKNOWN wrapped bottle. Printed in gold on lid of case “SANAX” FIRST AID CASE. Most of the contents, as well as the case, show the “SANAX” brand. Some contents are inscribed Burroughs Wellcome & Co. (Australia) Ltd., flagstaff hill, warrnambool, shipwrecked coast, flagstaff hill maritime museum, maritime museum, shipwreck coast, flagstaff hill maritime village, great ocean road, first aid items, first aid kit, emergency first aid, medical emergency kit, home emergency kit, industrial emergency kit, sanax company fitzroy melbourne, burroughs wellcome & co. (australia) ltd, thos. urquhart & son pty. ltd. melbourne, sanax first aid case, sanax first-aid emergency instructions, part 1 victorian factories regulations, tabloid medical supplies -
 Melton City Libraries
Melton City LibrariesPhotograph, Bruce Myers, 1931
My Story by Bruce Myers – June 2001 Arthur Bruce Myers was born on Wednesday morning on the 29/4/1925 at Kelvin Grove Hospital Bacchus Marsh. Background Information: Prepared by Niece Wendy Barrie. The early life of Bruce Myers “Burnbank” Ballarat Road Melton. The family home was built by his grandparents Ann nee Dowling and Henri Miers in 1867. His father Frederick was born in 1877 in Melton the youngest of four boys. Bruce the fourth son of Frederick and Martha, brother Frederick the eldest was followed by Marjorie and Edna. His brother Max was the youngest child. Father Frederick Myers attended Melton State School No 430 enrolling in 1881 and leaving in 1888 gaining his Merit Certificate No 116343. Bruce enrolled in July 1931 and completed and gaining his Merit Certificate in 1937. In 1938 he travelled to Melbourne Boys High School. Bruce was taught piano by his sister Marjorie, a respected Melton music teacher. He entered many Piano competitions and at the age of 10 winning the radio cup in the Junior Cavalcade at 3AW at Latrobe Street. At Melbourne Boys High School during his lunchtime was allowed to practice the piano in the basement for his recreation. He was pestered by another boy (name I have forgotten) a teacher intervened telling him to leave Myers alone. As a young child when listening to music he was able to on hearing it identify the key it was written in, due to his perfect pitch. I remember “Mum” Myers telling about the time they went to see Artur Rubeinstein at a concert, when Bruce was a small boy, it may have been on this occasion that he had noted the key of the piano composition. Bruce writes – In my early teens Max and I frequently accompanied the Williams boys, Wally and Jim on expeditions up the Toolern Creek near where the Gisborne exit now crosses it. The dogs would chase the rabbits into their burrows after placing nets over the burrows a ferret would be let in to burrow, much excitement would be involved in the rush to grab the rabbits as they bolted into the nets. In the same area I used too accompany Dad on an evening rabbit shoot (summer time). After the heat of the day the rabbits would emerge from their burrows at dusk. We would his behind the tree in silence, a mark contrast to the ferreting scene. Dad with the shotgun cocked would wait until 2 or 3 rabbits were close together then fire (Bang!). Hopefully killing two rabbits. They would have to be killed outright, otherwise they would run back into their burrows. Needless to say, one deafening shot ended the event, also it only cost one cartridge. Our only swimming pool was hole in the Toolern Creek at its junction with the blind creek at the eastern entrance to Melton. Dad swum there in the 1880’s teaching many of the youngsters to swim. Females never swum there to my knowledge. The dressing shed consisted of a 4 corrugated iron nailed to a wooden frame about 4 metres by 3 no floor or roof. We always walked the kilometre in our bathers anyway. The swimming hole once dried up leaving about 2 ft of mud. We Melton boys had so much fun fossicking around with our hands and feet and yanking out numerous eels, some very bid. I don’t know what happened to them all. No doubt Dad would have skun one or two for Mum to cook after cutting them up into short lengths. They used to jump around the pan when they were cooking. Dad accompanied by Max and I, frequently fished for eels in the Gillespie’s waterhole just below our place using a rod, line, sinker, hook baited with a worm, and a white floater so as to easily see when an eel was on the hook, so that it could quickly be pulled before it could anchor itself on and under water snag such as a tree root making it impossible to catch, or causing the line to be lost. At about the age of 8, I suddenly discovered amazingly easily means of movement. One day when I was riding the bike on rough bluestone road near the Presbyterian Church [Uniting Church] in Melton when the front fork broke and I landed on my right knee and right eye gashing both, the knee severely. I have carried the scars ever since. I started getting mobile by riding a scooter with good leg on the scooter and swinging the right leg, keeping is straight because bending it was too painful.Childhood photo of Brucelocal identities -
 Eltham District Historical Society Inc
Eltham District Historical Society IncFilm - Video (VHS), Diamond Creek Fire Brigade, c.1955-1987
Combination of three movie films. Movie One (1950s): 00:00 – 13:14 Black and white footage of Diamond Creek firemen practising in Diamond Street in the 1950s for forthcoming demonstrations of abilities. Mentions of Gordon Brandy and Joe Hislop Running out hoses from old hose reels along Diamond Street, Diamond Creek Displays from various brigades running out and connecting hoses. Also scenes from the 1950s of Diamond Creek Fire Brigade competing in various locations around Victoria and Tasmania. Mentions of Brigade members Dave Kidd, Bruce Hackett, Ron Kirkbride, Jack Marks, Graham Upton who are prominent in these events. Members of Kyneton Fire Brigade also present. Members competing in running out hose reels, connecting hoses togethers and to hydrants then climbing towers to direct water from hose or at a target hanging above the road. Diamond Creek members identified wearing a diamond on their chest and back. Includes scenes of Scottish pipe bands at the events and significant crowds of spectators. Footage of Mel Stone and Beryl Marks, Stan Redpath and Ron Kirkbride, then Ron Kirkbride and Eric Holt viewing flower displays. Film changes to colour at Diamond Creek oval for practice with fire engine entering oval. Members depicted include Bill May, Jack Sinclair, Jim Cox, Bob Beale, Dave Kidd, Bruce Hackett and Captain Clarrie Stone. Reverts to black and white in the 1950s where the Brigade joins forces with the Diamond Valley Community Hospital for a Gala Day on the Diamond Creek Oval. Changes to colour again, possibly same event and scenes of children on bikes and scooters or with prams and carts racing around the oval. Mention of young lad Brian Laurie who has his own fire truck. Dart throwing, pony rides. Scenes with Dr Don Cordner, Gus Lyons, Vic Cohn (?) and spinning wheel and Diamond Creek School children entertain a large crowd with Maypole dancing. Movie Two (1950s): 13:25 – 19:00 This black and white film was taken by a TV film crew in the 1950s depicts a typical call out for the Diamond Creek Fire Brigade. In this case the careless action of a member of the public throwing a lighted match from a car, which can cause extensive damage. Footage features the Shire of Eltham War Memorial tower at Kangaroo Ground before it was modified with a fire spotter’s cabin. Discusses fire spotting operations from the tower. Shows a fire spotter walking around the top of the tower. A fire is detected, and the information is relayed to the nearest fire station, in this case, Diamond Creek. The telephone call is received, and the alarm sounded. Captain Clarrie Stone and firemen May and Shaw leave their workplaces and prepare for action. Scenes of running across the Main Hurstbridge road showing the shops (Shell service station and Chemist prominent). Scenes entering the fire station which has a pictorial warning covering the entire door “Only you can prevent forest fires – If you’re careless – we’re homeless!” Eric Holt pinpoints the location of the fire while Captain Clarrie Stone and Fireman Shaw take note. The advance vehicle (an FE Holden ute, rego GTE-696) leaves to assess the extent of the fire. Having assessed the fire, Fireman Shaw communicates with base showing radio with call sign VL3JZ. Eric Holt takes the call. In the meantime, Captain Clarrie Stone and Fireman Shaw undertake some limited action to address the fire. Firemen Bill May, Jim Bates and Hugh Bar (?) man the tanker. A photo portrait of Queen Elizabeth is visible hanging on the wall. They are later joined by Firemen Jim Cox, Eric DeBuse (?) and Jack Marks. The tanker is seen departing the station and diverging off before the bridge. Captain Clarrie Stone and Fireman Shaw are seen pumping water on the flames with hand pumps when the tanker arrives. The hose is unreeled, and water turned on the flames. Jack Sinclair joins the action. Jim Cox directs water to the high stuff. The fire put out, Jack Marks and Eric DeBuse wind in the hoses and the team head back to town. It’s peaceful again at the memorial tower. Movie Three (1969-1987): 19:14 – 34:34 Colour film “Fired with Dedication”, Country Fire Authority Victoria, produced by I.L. Wadeson, Commentary by A.M. Hem. Credits with CFA Victoria emblem and then placed over a view of an old-style ladder engine. Opens with the scene of a fire engine outside the Diamond Creek Fire Station then various trophies reflecting the competition success of the brigade in various track and disciplined events. Two trophies shown of particular pride to the brigade were for first place in the Torchlight Procession at the State Championships in Mildura in 1986 and also at Swan Hill in 1981. Still photo scenes of ex Captain Clarrie Stone, Brigade Captain for 21 years; ex Captain Jack Marks, 10 years; ex Captain Ian Douglas, 10 years. Cuts to scene of radio control room, January 1969, and news of a fire on the northern side of the township of Diamond Creek. With scenes of flames in bush, the narration explains that until the early 1960s the area was an orchard district which protected the town against the savagery of bushfires. But due to competition from other areas more suitable for orcharding and easier transport to Melbourne the district could no longer remain competitive, and orchards were replaced by grassed areas, which together with the bush areas were a feeding ground for fire. On 8th January 1969, high temperatures and strong north winds, were, with the carelessness of some individual all that was necessary to produce the worst fire the district had seen. Cuts to scene of blackened fields and cattle - Hundreds of hectares of grass land were blackened, and cattle had to be transported to other areas for agistment. Scene of destroyed buildings in the township – 13 houses and the public hall in the town were destroyed as was the theatre equipment which was owned by the fire brigade. The Church of England Hall and bell tower were badly damaged. The whole town could have been burnt out but for the determination, skill, and courage of the Diamond Creek Fire Brigade. Scenes of all that was left of the home on the hill on the west side of the Church of England. Also, the remains of the old Pisy (?) home on the top of the same hill near Lambert Street, and the ruined Crocker home. Cuts to a scene in the mid-1970s to mid-1980s of a house fire in Haley Street attended by the Diamond Creek Fire Brigade. Although the house was severely damaged, it was saved. Mentions that whilst assistance is appreciated, in some circumstances, those doing so are not properly dressed for fighting fires. Breathing apparatus is a must in structure fire attack. Next scene (either on Mangarook or Coventry oval) showing off four Diamond Creek Fire Brigade efficient and very expensive firefighting units. Features a forward control vehicle Toyota 4WD used for conveying task force personnel to the required areas; a Hino Model 3.2 tanker, diesel powered and carries 3,000 litres of water and has a 16 HP petrol driven pump which delivers 900 litres of water per minute; an International tanker (registration TCM-418) which carries 3,000 litres of water with pumping capacity of 600 litres per minute. The Ford diesel powered pumper (registration MXE-754) is a well-equipped vehicle with a water capacity of 1,000 litres and capable of pumping 1,900 litres of water per minute from the main pump, has many lockers which hose equipment such as breathing apparatus and various types of hose nozzles and foam making equipment. The vehicle carries 360m of 64mm diameter hose which can be laid out from the rear lockers and a portable lighting plant, an Oxy Viva resuscitator to revive smoke inhalation victims and forcible entry tools to gain access to structure fires. Views of the main pump and control panel on the vehicle. As well as the main pump, the vehicle is equipped with an auxiliary pump which allows the facility to pump whilst moving. Fire fighters must undergo constant training and hone their skills, Scenes of a training exercise using the pumper to pump from static water. First, the short lengths of suction hose are coupled, a strainer fitted to ensure debris does not foul the pump. Gauges must be constantly monitored to ensure manageable water pressures are maintained. Pressures are normally controlled to allow two fire fighters to work at each nozzle outlet. Two nozzles are tested, one adjustable jet fog type which is used on flammable gasses or within a structure fire to absorb heat. A straight jet nozzle to project water long distances to protect exposed surfaces close to a fire radiated heat. The pumper is quite a versatile vehicle in handling structure fires, but it also carries specialist equipment needed in containing hazardous chemical incidents. Cuts to scene of parade – the Diamond Creek Fire Brigade has with other neighbouring brigades participated in most town fairs and earns the respect of the watching public. It can be seen why this brigade has been so successful at disciplined contests. Views of Plenty Fire Brigade Road Rescue unit which is equipped with the “Jaws of Life” Scenes of athletic competitions – many neighbouring brigades indulge in friendly but keen competition at the Diamond Creek Town Fair. The young are also encouraged to participate in all aspects of Junior Fire Brigade activities and become tomorrow’s generation of volunteer fire fighters. Scene of the 1986 Diamond Creek Town Fair which was the last time veteran Captain Clarrie Stone BEM marched with the brigade. Clarrie was awarded the British Empire Medal for his service to the Country Fire Authority. Also, scenes of vehicles in the parade. Cuts to scene of brigade members in drill formation for inspection by Acting Chief Harry Rothsay (?) on the occasion of the opening of the new fire station extensions on August 29, 1987. Rudy Libel (?) Captain at the time. Scenes of crowds including many dignitaries of neighbouring brigades present including Lieutenant Gordon Grandy (who came down from Queensland for the occasion) and ex-Secretary David Kidd and wife Betty, also ex Captain Clarrie Stone and Mrs Nel Stone, a life member of the Ladies Auxiliary, the Reverend Jock Ryan, son of J.L Ryan, founder of the Diamond Creek Fire Brigade, Foundation Captain of the fire brigade, Keith Bradbury and Mrs Bradbury. Pauline Dick accepts a community service award for services to the CFA. Recognising over 47 and a half years of service, a presentation is made by Mr Neil Marshall, Acting Chairman of the CFA to ex Captain Clarrie Stone with response by Clarrie. Other members of the official party include Cr. Martin Wright, Shire President Wayne Phillips and local Member of Parliament, Mrs Pauline Toner. Ex foreman John Bennett is presented with a life member’s awards by Captain Rudy Libel. The camera also catches Gwen Cox, Jean Ryan and Bessie Layton (?) Provides historic footage of people, places and equipment and a record of the worst fires expoerienced in Diamond Creek in 1969BASF Standard Quality SQ E-180 VHS dubbing (poor quality) of three films Converted to MP4 file format 0:34:38, 1.85GBOn label: "Donation - August 2000 Diamond Creek Unit Old films made up from Fire Brigade shows at competitions - also Kangaroo Ground Tower being used"video recording, diamond creek fire brigade, 1986 diamond creek town fair, a.m. hem, acting chief harry rothsay, athletic competitions, beryl marks, bessie layton, betty kidd, bill may, bob beale, brian laurie, bruce hackett, captain clarrie stone, chemist, church of england hall, clarrie stone, clarrie stone bem, country fire authority victoria, coventry oval, cr. martin wright, crocker home, dart throwing, dave kidd, david kidd, diamond creek, diamond creek fire station, diamond creek oval, diamond creek school, diamond creek town fair, diamond street, diamond valley community hospital, dr don cordner, eric debuse, eric holt, fe holden ute, fire damage – buildings, fire spotter, fire spotter’s cabin, fire station extension, fired with dedication (film), firefighting units, fireman shaw, firemen jim cox, ford pumper, foundation captain, gala day, gordon brandy, gordon grandy, graham upton, gus lyons, gwen cox, haley street, hino model 3.2 tanker, house fire, i.l. wadeson, ian douglas, international tanker, j.l ryan, jack marks, jack sinclair, january 1969, jaws of life, jean ryan, jim bates and hugh bar, jim cox, joe hislop, john bennett, kangaroo ground, kangaroo ground tower, keith bradbury, kyneton fire brigade, lambert street, main hurstbridge road, mangarook oval, maypole dancing, mel stone, mildura 1986, mrs bradbury, mxe754 vic registration, neil marshall, nel stone, orchard district, oxy viva resuscitator, pauline dick, pauline toner mp, pisy home, plenty fire brigade road rescue unit, pony rides, radio control room, reverend jock ryan, ron kirkbride, rudy libel, shell service station, shire of eltham war memorial, shire president wayne phillips, spinning wheel, stan redpath, state championships, swan hill 1981, tcm418 vic registration, torchlight procession, toyota 4wd, trophies, vic cohn, victorian bushfires - 1969, vl3jz -
 Eltham District Historical Society Inc
Eltham District Historical Society IncBook, Percy Leason: an artist's life by Margot Tasca, 2016
"Who would have thought that a boy born in 1889 from the Victorian Mallee would become a successful artist on New York’s Staten Island? This finely illustrated, exhaustively researched and beautifully written biography on Leason features the artist’s entire career as a painter and cartoonist renowned for his depictions of Australian society in the 1920s and 1930s. Leason’s story is a poignant one tracing his beginnings as a cartoonist, to the bohemian Melbourne art scene in the early 20th century, to his involvement in the artists’ camps of Eltham, to his important series of portraits of Lake Tyers Indigenous Australians, and his eventual move to the US where he has been acknowledged as making an enormous contribution to the New York arts scene. This story, as yet untold, fills a gap in the history of art in Australia and offers a new perspective on Australian art in the first half of the 20th century." - Thames and Hudson website A NEW HOME IN ELTHAM Once they had settled back into Melbourne, Perry and Belle began to look for a place to make a permanent home. Having enjoyed the bush setting of Mosman, they decided to explore the rural fringes of Melbourne. Each weekend they packed a picnic and travelled to the towns in the nearby hills - such as Ferntree Gully, Sassafras, Lilydale and, of course, Cockatoo Creek. Eventually deciding these places might be a little too far from The Herald office, they searched closer to the city. The Heidelberg and Box Hill regions that had inspired his old teacher McCubbin, had become busy, urban areas but further east, towards Warrandyte and Templestowe, there were still large tracts of bush. Finally they settled on Eltham, an area Percy knew very well, having often painted there with Jock Frater. Perry's old friend Dick McCann and his wife Margery had also settled in Eltham. The township was fifteen miles from Melbourne and serviced by an electric train that went to the central Melbourne station of Flinders Street, near where The Herald offices were located. Eltham was a small village in 1925, separated from Melbourne by the Yarra River, and surrounded by orchards and large tracts of bush. Small farms dotted the landscape and the main businesses revolved around ironmongers, blacksmiths, and farming supplies. Of particular appeal to artists was Eltham Park, a large expanse of bushland bounded by the Yarra River on the south side and the Diamond Creek on the east. The park included a playing field that was busy on weekends with cricket or football matches, but for the rest of the week it was mostly empty and an ideal place to paint. The scenery there provided the inspiration for many paintings by Leason, Meldrum and other artists such as Colin Colahan and Peter (A.E.) Newburv. The Leasons found a rundown old farmhouse on four-and-a-half acres of land in New Street, now known as Lavender Park Road. The site was splendid, at the top of a gentle slope which gave panoramic views east to the Dandenong hills, south over the Templestowe orchards and north to Kinglake. The front lawn was taken over by onion grass (or wiregrass as Leason called it) and scattered about the property were many wattles and gum trees. Aloe cacti covered much to the front of the house, while old quince and lucerne hedges separated the house and out-buildings from a rundown apple orchard. Here they would build a new home. ·with financial assistance from The Herald, Leason bought the property and immediately commissioned an architectural firm to design a new house in the popular bungalow style of the time. The old farm house was demolished but Percy saved the siding boards, bricks and corrugated iron for the outbuildings of his new home. The new house was a two storey, triple brick with a large, gabled, terracotta tiled roof. It was situated at the very top of the slope. The paint and varnish were barely dry when the family moved in during the summer of 1925-26 and the fumes were overpowering in the heat. Despite the house being wired for electricity, power poles had not yet reached the area and initially the family had to rely on kerosene lamps and candles. When electricity did arrive, Leason reflected on the community's reception of electricity at the expense of the old growth gum tree corridors in his cartoon, Electricity comes to Wiregrass. The family had now grown to seven. Jack was nearly nine, Jean was seven, Marjory was four, Nancy was two and the baby Patricia was seven months old. Jack and Jean were enrolled in the local primary school down the hill. A retired farmer, Jock McMillan, came to live on the property and help out with the general maintenance. Jock built himself a shack and Belle provided him with meals. He was kept occupied building structures around the property·, such as the garage, the outside toilet, garden beds, trellis arbours and a number of ponds. The elderly, bearded Scotsman with his old hat and baggy pants also provided the inspiration for one of the characters Leason regularly included in his cartoons. Like Leason, Jock smoked a straight stemmed pipe. A neighbour was employed to help Belle with domestic chores, and so the family settled down to live comfortably in their new Eltham house. Two dogs, Maginary and Wodger, completed the large and vibrant household. “Percy Leason; an artist’s life” by Margot Tasca, Thames & Hudson Australia Pty Ltd, Port Melbourne 2016, pp 63-64 Hardback Bookpercy leason, margot tasca, biography, artist, landscape -
 Melton City Libraries
Melton City LibrariesNewspaper, 'Call for new members or society maybe be history, 2003
Mary Tolhurst M&DHS - March 29th Dunvegan Willows Park Melton 1992 Ladies Oral History Day Graham Minns President Ray Radford MC Sound recording transfer to CD 2011 by Tom Wood Edited typescript by Wendy Barrie 2013 I was born in Rockbank, and when I was five years old moved to Toolern Vale and started and finished school there. Toolern Vale only consisted of the Store, Post Office and shop, where you could buy your fodder, and pollard supplies, the Hall, the little Church and the bluestone School. The School changed shape three times from the 1800s[1869] til the time I went there. There was four generations of my family that went there and it was destroyed by fire in 1965. Marjorie nee Myers Butler. Yes, I remember along with it your lovely Ronisch piano. Mary, quite true! Marj what you say about the Ronisch piano. When I came the age to learn music my mum and dad couldn’t really afford it, but still what parents do for their children. They had Marj go along with them and pick this lovely Ronisch piano. It was known round the district. Everyone commented about the loss that lovely piano. After leaving school it was war time, 1939, then it was work, When I was 7 year old I was put out into the cow yard. In 1940 when the soldiers were going away our milk was confiscated it had to go to Bacchus Marsh. It used to go the Sunbury to be brine cooled and then go to Melbourne. Then they took it then to the Lifeguard Milk Factory at Bacchus Marsh. It had to go as condensed milk to the soldiers. This year is 50 years of the Land Army. I was an unofficial Land Army but they still kept check on me. I went onto married life and I followed the cows right through [howls of laughter] and we went on until the 1965 fire. That’s when we got out of the cows. Marjorie asks, was Granny Watts your grandmother or great grandmother? Mary: She was my great grandmother, the midwife of Melton. The 1965 fire started ¾ of a mile above our place, Frank Ryan’s sheds were burnt and his house was saved, then it wiped the School out, the Hall, the Church the Post Office and Store and little house that was Charlie Charlton’s in the early days. Mrs Wilson’s place was saved by the Fire Brigade by pulling boards off the side, and from there it went over the hill and it was stopped at the Rockbank Railway Station. If it had of got over the railway they said it would have gone into Werribee. A lot was burnt out in that strip. Mary nee Nixon Collins: 18 houses burnt that day. Audience question, did Melton get burnt that day? Ray: No. It came down through the Toolern Vale road and cut across about a mile and a half from the cross roads at Toolern Vale from north westerly to the south east and cut through over the Keilor road. Mary: It came in across the creek at Funstons in Toolern, then through Jim Minns. Dorothy was it your place then [nee Knox Beaty] to Ken Beatty’s and from there it went through to Doug McIntosh’s and to Cockbills and the wind changed and it came across to the railway line, and that is where they stopped it. [the cause of the fire was controversial, they had been burning off the night before and there was some talk of someone starting it. It was very hot and very strong wind, it was a terrible day] Ray: When the fire went through McIntosh’s they had a haystack on the north side of their house and the haystack got caught and the fire burnt a hole through the side of the house and the boys pyjamas on the bed. The house was saved. It came through like and express train roaring at you, I was at McIntosh’s when it went roaring past. You couldn’t see, dust and ash and tremendous heat. The fire started about 12 o’clock Jack [husband] said to me, fire, I said where, where? Just up the road, what have I got to do? and he went out and he had gone to the fire and left me. I tried to get the animals and I put out buckets of water, putting the buckets of water out saved my life. Chas Jones and another friend of his came in and they picked up the buckets of water, I thought I had better get out because the fire was on the haystack up the paddock and when I went to go out through the north side of the house and couldn’t get out, I’ll go through the front gate so I went around the other side of the house. I got caught there and Chassy Jones and his friend came round carrying the bucket of water and I panicked. He threw the bucket of water over me. Well that is what saved my life because I was damp, whenever we tried to leave the ball of fire came over me and over my shoulder and my hair was scorched. Chassy Jones lost his truck and Keith Watt his big truck because he had the water tank on it and they couldn’t get out of the yard. Granny Watt’s house, the first private hospital had condemned and Jack and I pulled it down and had it moved up to Toolern and had it in the yard a fortnight and it was all burnt and we didn’t get the shed we wanted. Every 13 years right up until Ash Wednesday fires, there has always been fire close at hand. The 1952 fire went down the back of the house, the 1965 fire took the house, and the house that I live in now, it is the third house that has been on that spot. When the Hunters owned it, Mrs Hunter was nearly burnt in her bed. They had a 13 roomed house. In 1924 the house burnt down, and there was another house was built there and that was the one that burnt down. Edna: So Mary built a brick veneer house. Marjorie: like the three little pigs [laughter] Mary Tolhurst member of the Melton & District Historical Society in the Melton and Moorabool Leader local identities, local special interest groups -
 Melbourne Tram Museum
Melbourne Tram MuseumMagazine, Yarra Trams, "The Wire", 5/2011 to 1/2015
0 - No 9 - 1/4/2011 - Rhinos on skateboards, Did you know, Spencer St works, 00 - No 10 - 19/4/2011 - safety boards, busting ghost routes, works alerts, travelling without tickets, launch of a new tram network maps and compliments received from customers. .1 - No. 11 of 3/5/2011 with the revised Yarra trams logo, traffic priority, work over Easter in Spencer St at Bourke and Collins St, Good Friday appeal, safety, passenger feedback and future works. .2 - No. 13 - 31/5/2011 - new uniform, cleaning, CEPR, trackwork - Fitzroy St, Northcote, Rhino, Carlton Control. .3 - No. 14 - 15/6/2011 - Haymarket Roundabout, accessibility, maintenance, CSE. 3a - No. 16 - 19/7/2011 - Managers on the move, Trevor Jones, Yarra's vision, Richard Ch'ng and Rhino update. .4 - No. 17 - 2/8/2011 - High St Westgarth trackwork, Swanston St, IMF CEO visit .5 - No. 18 - 16/8/2011 - Performance benchmarks met, Preston Workshops, repairs to 3018, tram signal priority. .6 - No . 19 - 30/8/2011 - New E class trams, routes "a" or "d", TramTracker in shelters, police, fare evasion .7 - No. 20 - 15/9/2011 - Football trams, Superstops, Bridge Road, Rhinos. .8 - No. 21 - 27/9/2011 - CEO's journey to work, accessibility, increased patronage, E class. .8a - No. 22 - 11/10/2011 - Minister Mulder visit, E class, Customer experience, Elizabeth Kerdelhue Corporate Affairs Director, flood indicator in Wellington Parade, Keolis - Orleans and PTV coming your way. .9 - No. 23 - 25/10/2011 - forthcoming royal visit, opening for Footscray Road extension, Rhinos, Stockholm .10 - No. 24 - 8/11/2011- Royal visit, photos, Z3 158, route 86 works in High St. (see htd5043i21 for a image from an unknown newspaper of the actual event - features Z3 158.) .11 - No. 25 - 22/11/2011 - new staff guide, Gold Coast tram line, Macarthur St, overhead, fund raising, route numbering update. .12 - No. 26 - 6/12/2011 - Swanston St Superstops, Newmarket bridge strikes, rhinos. .13 - No. 27 - 20/12/2011 - Christmas carnival, Lenny Bates, portable crossover, uniforms. .14 - No. 28 - 17/1/2012 - Passing of Len Bates, Myki, Gardiner railway station. 14a - No. 29 - 31/1/2012 - Southbank depot, patronage, myki, think like a passenger, fatigue management, .15 - No. 30 - 15/2/2012 - visit of Keolis, SNCF people, list of Executive leadership team with photos, Swanston St works, Myki introduction. .16 - No. 31 - 29/2/2012 - patronage up, tram postage stamps, Myki, rhinos. .17 - No. 32 - 14/3/2012 - St Kilda Rd trackwork, fund raising, Southbank Depot extensions, Myki, driving conditions, grand prix. .18 - No. 33 - 30/3/2012 - introduction of the PTV, end of MetLink and Transport Ticketing Authority, changes in management structure, trackwork, Gold Coast tramway and Keolis. .19 - No. 34 - Dr Jake - Royal children's Hospital super stop, route 96 - Premium line. .20 - No. 35, 2/5/2012 - Revision of Rules, trackwork in St Kilda Road and Elizabeth St, Myki, safety - Zero Harm. .21 - No. 69 - 25/9/2013 - Passengers paying their way, E class update, Mal Ashworth retires, progress report, feedback, new chime on trams. .22 - No. 70 - 9/10/2013 - Art comes alive, tram 925, driver simulator at Preston Workshops, E class project, 90th Glen Huntly. .23 - No. 83 - 23/4/2014 - Screen time for trams, new PIDs on B class, assistance animals, Operations Centre, Preston Workshops, Electrical log sheets to SLV. .24 - No. 89 - 23/7/2014 - punctuality, refresh of network map (fold-out map), women drivers. .25 - No. 97 - 19/11/2014 - Revitalising route 96, Keolis news, free tram zone, guide dogs. .26 - No. 99 - 17/12/2014 - Accessibility week, new uniform top for CSE's, free tram zone, world trade centre stop upgrade, heat stress, Art tram 158. .27 - No. 100 - 14/1/2015 - Route 96 complete, New Years eve free travel, fare compliance, patronage down, .28 - No. 12 - 16/5/2011 - Gold coast tramway, performance dashboard, tramworks and the rhino .29 - No. 16 - 19/7/2011 - Depot managers, tevor jones, record patronage, vision, rhino .30 - No. 17 - 2/8/2011 - High St Westgarth works, Duncan Smith, David Clarke Training, Swanston St works, and Preston Workshops . .32 - No. 39 - 28/6.2012 - maintenance, Emmanual Sorin, transformation, fare evasion, and Combino in Potsdam. .33 - No. 105 - 25/3/2015 - Grand Prix, Elgin and Lygon upgrade, Camberwell Junction, PTV hub, overhead. .34 - No. 78 - 12/2/2014 - January heatwave, Australian Open, Mark Wild of PTV, and Curt Skinner - voice in Channel 10 series Get Ace .35 - No. 81 - 26/3/2014 - drug and alcohol testing, zero harm, Victoria Bridge works, Keolis, relations with Toronto, Collins St safety and incident on a route 57 tram. .36 - No. 109 - 20/5/2015 - Goodbye Z class, Hello E class, Kew Depot centenary, drug & alcohol reminder, passenger satisfaction, Anzac day, B class life extension. .37 - No. 110 - 3/6/2015 - 3rd W class tram back grom Bendigo, employer of the year, CSE's go digital, Camberwell depot, Queensbridge tram and bus stop and Tram Hub preview. .38 - No. 115 - Feb. 2016 - 12 page centre stapled - New Year's eve services, spike the rhino is back, Burke Road level crossing removed, more E class, safe network in 2106, tennis, customers happy, New Preston depot, Farewell to Clement, Z class 40 years on network.Demonstrates Yarra trams staff newsletters.Set of 34 Yarra Trams internal newsletter "The Wire", All A4, printed in full colour. All four pages unless noted otherwise, full colour, performance snapshot on front cover.trams, tramways, yarra trams, traffic control, trackwork, spencer st, fund raising, operations, rhinos, carlton control, high st, haymarket, preston workshops, e class, route numbers, bridge road, wellington parade, ptv, royal visit, footscray road, gold coast, macarthur st, swanston st, superstops, newmarket, gardiner, burke road, level crossings, railway squares, myki, metlink, tickets, route 96, rules, st kilda road, elizabeth st, tram 158, tram 925, glen huntly depot, simulator, b class, opeations centre, art trams, patronage, kew depot, new preston, queensbridge, w class, bendigo, map -
 National Wool Museum
National Wool MuseumClothing - 1984 Los Angeles Olympics Men's Opening Ceremony Shirt, c. 1984
On the 1984 Los Angeles Olympic Uniforms donator Doug wrote- During the 1980s the Australian wool industry was at its most prosperous times with record numbers of sheep producing wool receiving ever increasing values due to the success of the Reserve Price Scheme, and the overall guidance of the Australian Wool Corporation (AWC). As a humble technician, my role was a low profile newly created position of “Controller, Technical Marketing” where wool was to be marketed on its technical properties, as distinct from the “Product Marketing Group” which exploited trhe traditional high profile approach of marketing wool;s superior fashion attributes. The Woolmark was the tool central to this approach. When the forthcoming Los Angeles Olympic Games was announced, the Product Marketing Group seized upon the chance to show the world that we could make top fashion garments and display them on our elite athletes on the world stage. A concept was launched using a contemporary top designer, Adel Weiss, with the most exclusive fabrics and knits available, and all with a lot of hype. This launch failed dismally for the following reasons- - The designer did a wonderful job presenting an excellent fashion range on perfect skinny models. The AOC however wanted a uniform which had an obvious Australian appearance when fitted to elite, and frequently muscular, athletes. - The fabrics chosen did not reflect the performance required by travelling athletes, there was no recognition of the need for ‘easy care.’ - There was no recognition given to the problem of measuring, manufacturing and distribution of a range of articles when the selected athlete could be domiciled anywhere in Australia. - There was no appreciation of such historical facts as Fletcher Jones, who had been unofficial suppliers dating back to the 1954 Olympics in Melbourne, and the Fletcher Jones board member, who was also an AWC board member, and was not in favour of the change. The project passed from Product Marketing to Public Relations, a big spending off-shoot of the AWC Chairman David Asimus, and due to the day to day operations of the project was passed to me and PR took care of the financial matters. The first task was to meet with the AOC and find out exactly their requirements. This lead to the production of a design and manufacturing brief, cointaining exact time lines for each event required to ensure an appropriate uniform on every athlete chosen to represent his/her country on the date given for the Opening Ceremony in Los Angeles. Working backwards the timeline becomes- 1. Noted the exact date of the Opening Ceremony. 2. Estimated the date for distributing completed garments to each athlete. 3. Estimated the time span available for measuring each athlete and commence making each component of the ensemble to the individual measurements of each athlete. 4. Decided the date for making the final choice of uniform design concept. 5. Decided the date for distribution of the design brief to selected designers. These five steps were spread out over a two year period. The Commonwealth Games occur midway between each Olympic Games, work on the Olympic uniform commences the week after the Commonwealth Games closing ceremony and MUST be ready by the prescribed day two years hence. The project also had to remain cognisant of trade politics existing within the span of the task, as well as the temperament of designers in general. It is no overstatement to say that in the past every designer in Australia believed they could, and should, be chosen to design the Australian Uniform. The final choice of designer almost always faced criticism from the fashion press and any designer who had been overlooked. However, with the contenders receiving an exacting brief the numbers of serious contenders greatly reduced. The Los Angeles Olympic Uniforms. A further reason for the AWC bid failure to design the LA uniform was that the AOC had already chosen Prue Acton to design it. This was based on her proven performance during previous games as she had a talent for creating good taste Australiana. Her design concepts also considered the effect when they were viewed on a single athlete as well as the impact when viewed on a 400 strong team coming on to the arena. A blazer trouser/skirt uniform in bright gold was chosen for the formal uniform. It was my task to select a pure wool faille fabric from Foster Valley weaving mill and have sufficient woven and ready within the prescribed timeline. The trouser/skirt fabric selected was a 60/40 wool polyester plain weave fabric from Macquarie Worsted. This fabric had a small effect thread of linen that was most attractive when dyed to match some eucalyptus bark Prue had brought back from central Australia. For the Opening Ceremony uniform, Prue designed a series of native fauna, a kookaburra for the men’s shirt and a pleated skirt with a rural scene of kangaroos, hills and plants. This presented an insurmountable printing challenge to the local printing industry as it had an unacceptably large repeat size and the number required (50) was also commercially unacceptable. The solution was a DIY mock up at RMIT and the employment of four student designers. The fabric selected for this garment was a light weight 19 micron, pure wool with a very high twist yarn in alternating S and Z twist, warp and weft. This fabric proved to be the solution to a very difficult problem, finding a wool product which is universally acceptable when worn next to the sin by young athletes competing in the heat of a Los Angeles summer. Modifications to this fabric were developed to exploit its success when facing the same problem in future games. Garment Making- The most exacting garment in the ensemble is the tailored blazer, plus the related trouser/skirt. Unfortunately tailoring athletes that come in various shapes and sizes such as; - Weight lifters develop an enormous chest, arms and neck size. A shirt made to a neck size of 52 would produce a shirt with cuffs extending well beyond the wearer’s hands. - Basketball players are up to 7 feet tall and garments relying ona chest measurement grading would produce a shirt with cuffs extending only to elbow length. - Swimmers develop enormous shoulders and slim hips, cyclists by contrast develop thighs I liken to tree trunks and a uniform featuring tight trousers must be avoided at all cost. Suffice to say many ensembles require specialist ‘one off’ treatment for many athletes. Meanwhile there is a comfortable in between group who can accept regular sizes so you can cater for these by having back up stock with plenty of built in contingencies. Athletes may be domiciled anywhere in Australia, this creates a fundamental problem of taking their measurements. The Fletcher Jones organisation was key to answering this problem due to their presence in every capital city, as well as many provincial towns around Australia. Each athlete on being selected for the Olympic Team was simultaneously requested to visit their nearest Fletcher Jones shop. The standardised measurement data collected was shared with the other manufacturers, e.g. Pelaco Shirts, Holeproof Socks and Knitwear, Maddison Belts, and even Hush Puppy Shoes. As the time for the Games approached the AOC made arrangements for combining meeting of all. Selected available athletes at the Australian Institute of Sport, Canberra, where, among other things, they were fitted and supplied with their uniform. The method evolved as follows.Men’s cream coloured button up, collared shirt. Images of a kookaburra have been printed onto the shirt, a single kookaburra on the left breast and a pair of kookaburras on the reverse of the shirt. The kookaburras are printed in a brown tone to complement the cream colour of the fabric.On tag - FMaustralian wool corporation, 1984 los angeles olympics, olympic uniforms, men's uniforms, sport, athletes -
 Flagstaff Hill Maritime Museum and Village
Flagstaff Hill Maritime Museum and VillageEquipment - Sharpening Stone, ca 1878
The sharpening stone can also be referred to as a whetstone, oil stone or honing stone. It is a well-worn double-sided sharpening stone retrieved from the wreck site of the Loch Ard. It is used to grind and hone the edges of metal blades and tools. ‘Natural’ sharpening stones like this one are quarried from ancient sedimentary rock that has metamorphosed from clay and volcanic ash to produce garnet crystals. Most modern stones are artificially produced, or ‘bonded’, abrasive stones, made by fusing clay and metal powder under heat and pressure. The softer yellow Corticule stone is found in thin vertical veins running through the more plentiful Belgian Blue rock. Coticule is a fine-grained and dense material that ‘cuts’ metal slowly but to a superior standard of sharpness and finish. The relatively coarser Belgian Blue is stronger and ‘cuts’ more quickly, but with a less polished finish. A double-sided whetstone is therefore valued for its increased durability (the harder BBW ‘backs’, or supports, the softer Coticule), and additional utility (the fine ‘grit’ of Coticule complements the coarser BBW to meet a range of sharpening needs). The blue-grey base of this stone is thinner than the remaining yellow Coticule on top. This suggests that the majority of grinding and honing work it has done on board the ship was for larger tools, rather than on surgical or shaving blades. Its rounded or spherical shaping may also be related to the ‘tumbling’ action of the sea on the ocean floor. History of the Loch Ard wreck: The Loch Ard got its name from ”Loch Ard” a loch that lies to the west of Aberfoyle, and the east of Loch Lomond. It means "high lake" in Scottish Gaelic. The vessel belonged to the famous Loch Line which sailed many vessels from England to Australia. The Loch Ard was built in Glasgow by Barclay, Curle & Co. in 1873, the vessel was a three-masted square-rigged iron sailing ship that measured 79.87 meters in length, 11.58 m in width, and 7 m in depth with a gross tonnage of 1693 tons with a mainmast that measured a massive 45.7 m in height. Loch Ard made three trips to Australia and one trip to Calcutta before its fateful voyage. Loch Ard left England on March 2, 1878, under the command of 29-year-old Captain Gibbs, who was newly married. The ship was bound for Melbourne with a crew of 37, plus 17 passengers. The general cargo reflected the affluence of Melbourne at the time. Onboard were straw hats, umbrellas, perfumes, clay pipes, pianos, clocks, confectionery, linen and candles, as well as a heavier load of railway irons, cement, lead and copper. There were other items included that were intended for display in the Melbourne International Exhibition of 1880. The voyage to Port Phillip was long but uneventful. Then at 3 am on June 1, 1878, Captain Gibbs was expecting to see land. But the Loch Ard was running into a fog which greatly reduced visibility. Captain Gibbs was becoming anxious as there was no sign of land or the Cape Otway lighthouse. At 4 am the fog lifted and a lookout aloft announced that he could see breakers. The sheer cliffs of Victoria's west coast came into view, and Captain Gibbs realised that the ship was much closer to them than expected. He ordered as much sail to be set as time would permit and then attempted to steer the vessel out to sea. On coming head-on into the wind, the ship lost momentum, the sails fell limp and Loch Ard's bow swung back towards land. Gibbs then ordered the anchors to be released in an attempt to hold their position. The anchors sank some 50 fathoms - but did not hold. By this time the ship was among the breakers and the tall cliffs of Mutton Bird Island rose behind. Just half a mile from the coast, the ship's bow was suddenly pulled around by the anchor. The captain tried to tack out to sea, but the ship struck a reef at the base of Mutton Bird Island, near Port Campbell. Waves subsequently broke over the ship and the top deck became loosened from the hull. The masts and rigging came crashing down knocking passengers and crew overboard. When a lifeboat was finally launched, it crashed into the side of Loch Ard and capsized. Tom Pearce, who had launched the boat, managed to cling to its overturned hull and shelter beneath it. He drifted out to sea and then on the flood tide came into what is now known as Lochard Gorge. He swam to shore, bruised and dazed, and found a cave in which to shelter. Some of the crew stayed below deck to shelter from the falling rigging but drowned when the ship slipped off the reef into deeper water. Eva Carmichael a passenger had raced onto the deck to find out what was happening only to be confronted by towering cliffs looming above the stricken ship. In all the chaos, Captain Gibbs grabbed Eva and said, "If you are saved Eva, let my dear wife know that I died like a sailor". That was the last Eva Carmichael saw of the captain. She was swept off the ship by a huge wave. Eva saw Tom Pearce on a small rocky beach and yelled to attract his attention. He dived in and swam to the exhausted woman and dragged her to shore. He took her to the cave and broke the open case of brandy which had washed up on the beach. He opened a bottle to revive the unconscious woman. A few hours later Tom scaled a cliff in search of help. He followed hoof prints and came by chance upon two men from nearby Glenample Station three and a half miles away. In a complete state of exhaustion, he told the men of the tragedy. Tom then returned to the gorge while the two men rode back to the station to get help. By the time they reached Loch Ard Gorge, it was cold and dark. The two shipwreck survivors were taken to Glenample Station to recover. Eva stayed at the station for six weeks before returning to Ireland by steamship. In Melbourne, Tom Pearce received a hero's welcome. He was presented with the first gold medal of the Royal Humane Society of Victoria and a £1000 cheque from the Victorian Government. Concerts were performed to honour the young man's bravery and to raise money for those who lost family in the disaster. Of the 54 crew members and passengers on board, only two survived: the apprentice, Tom Pearce and the young woman passenger, Eva Carmichael, who lost her family in the tragedy. Ten days after the Lochard tragedy, salvage rights to the wreck were sold at auction for £2,120. Cargo valued at £3,000 was salvaged and placed on the beach, but most washed back into the sea when another storm developed. The wreck of Lochard still lies at the base of Mutton Bird Island. Much of the cargo has now been salvaged and some items were washed up into Lochard Gorge. Cargo and artefacts have also been illegally salvaged over many years before protective legislation was introduced in March 1982. One of the most unlikely pieces of cargo to have survived the shipwreck was a Minton majolica peacock- one of only nine in the world. The peacock was destined for the Melbourne 1880 International Exhibition. It had been well packed, which gave it adequate protection during the violent storm. Today the Minton peacock can be seen at the Flagstaff Hill Maritime Museum in Warrnambool. From Australia's most dramatic shipwreck, it has now become Australia's most valuable shipwreck artifact and is one of very few 'objects' on the Victorian State Heritage Register. The shipwreck of the Loch Ard is of significance for Victoria and is registered on the Victorian Heritage Register ( S 417). Flagstaff Hill has a varied collection of artefacts from Loch Ard and its collection is significant for being one of the largest accumulation of artefacts from this notable Victorian shipwreck of which the subject items are a small part. The collections objects give us a snapshot of how we can interpret the story of this tragic event. The collection is also archaeologically significant as it represents aspects of Victoria's shipping history that allows us to interpret Victoria's social and historical themes of the time. Through is associated with the worst and best-known shipwreck in Victoria's history. A sharpening stone is also called a whetstone, oil, or honing stone. The stone is a worn double-sided rectangular block with rounded corners. There is a clear delineation between its coarser Belgian Blue base (grey colour) and its finer Belgian Coticule face (yellow colour). It bears sedimentary encrustation over one-third of its surface. flagstaff hill, warrnambool, shipwrecked coast, flagstaff hill maritime museum, maritime museum, shipwreck coast, flagstaff hill maritime village, great ocean road, loch line, loch ard, captain gibbs, eva carmichael, tom pearce, glenample station, mutton bird island, loch ard gorge, sharpening stone, whetstone, oilstone, double-sided stone, belgian coticule, belgian blue whetstone, oil stone, honing stone -
 Federation University Historical Collection
Federation University Historical CollectionBook - Book - Press Clippings, Ballarat University College/University of Ballarat School of Visual and Performing Arts, 1993 - 1999, 1993
Blue cover book of press clippings. .1) 1993 - briar rabbit, brer rabbit, Libby Tanner, Lorrae Desmond, Cherry Orchard .2) 1994 - Bruce Widdop, Eureka, Rebellion, Aiden Fennessy, Steel Magnolias, Rumpilstiltskin, Tale of Two Cities, Peter Tulloch, Ring Round the Moon, Grainery Lane, Barnstorm Theatre, Rivers of China, Lord Wedgewood, Rick Chandler. James Charters, Matt Molony, Antoninino Atzori, Joseph, Len Bauska, Joseph and the Amazing Technicolour Dreamcoat, Damian Muller, Bert Labonte, Once a Catholic, Peta Brady, Fiddler on the Roof, King Richard III, The Seagull, Mr Men .3) 1995 - Point of Departure, Stags and Hens, Hansel and Gretel, Rob Knowles, The Would Be Gentlemen, SOund of Music, Barry Breen, The Bundle, Karl Hutton, Much Ado About Nothing, Len Bauska, Hamlet, Pajama Game, Peter Tulloch .4) 1996 Melissa Casey, The Wizard of Oz, Peter Tulloch, Libby Tanner, Rooted, Erard Concert Grand Piano, Atlantis. The Visit, Stella Axarlis, Me and My Girl, Our Country's Good, Three Billy Goats Gruff, Hold the Mayo, The Crucible, Chris Dickins, Stuart Pursell, Arts Academy .5) 1997 - Tempest at Loch Ard Gorge, Maelstrom, Angela Coad, Damian Muller, West Side Story, The Importance of Being Ernest, Mark Gambino, Brett Edgington, Cosi, Damian Muller, Leonard Bauska, Matthew Heenan, Amanda Sandwith, Bacchae, Richard DiGregorio, Roger Woodward, Peter Tulloch, Gavin Fenech, Bruce Widdop, Lola Montez, Tim Haymes, Tina Ford, Ross Jones .6) 1989 - Graeme Bird, Leanne Lettieri, Ballarat Symphony Orchestra, Christopher White, David Addenbrooke, Bruce Widdop, Chris Betts, John Garland, Allan Mann, John Sharpham, David Forrest, Warwick Stengards, Jan Davis, Wendy Morrison, Equus, Scott Cameron, Bryan Trueman, Peter Blizzard, Andrew Burnham, Peter Pilven, Chalk Circle, Tsou Nan-Chien, Ten Little Indians, Doug Wright, Stellarc, Chris Betts, Eric Lovett, Bob Allan, Doug Wright, Kaspar, Bill Levis, The Removalists, Liz Poklar, Goldfields Print Award, Margaret Sulikowski, Kathy Gamble, Maria Froia-Crump, Ian Hemmingway, Geoff Wallis .7) 1989 - Shirley Randall, Fred Sulikowski Fargher, Richard Jeziorney, Shane Lee, Neville Philpott, Val Lehman, Bill Levis, Hamp, Peter Ford, Shane Lee, Richard Akers, Peter Blizzard, Debbie Fraser, Shane Lee, Away, Genevieve Lacey, Pauline Coutts, Tsou Nan-Chien, Petrus Spronk, Debbie Fraser, Chris Betts, David Addenbrooke, Alan Peascod, John Crump, Deb Rosser, Michael Cook, Bruce Widdop, Jenny Trickey, Jennifer Marshall, Stellarc, Carboni, Stuart Matteson, Peter Sargeant retirement, Lyn Conellan .8) 1990 - Micehelle Tuddenham, Pauline Coutts, Anthony Horton, Claire Dale, Kryal Castle, Howard Tostivan, Simon Buckle, Blitz, STelarc, Hitz of the Blitz, Doug Wright, Nerissa Heath, Mieke Glickson, Ruth Greenburg, Peter SParkman, Allan Mann, Rachel Appleton, Michelle Tuddenham, Romeo and Juliet, Jennifer Pacey, Felicity Hay, Kristen Boys, Shane Lee, Norm Strange, Demolition Job, Merran Lisette, Charlotte's Web, Merran Hedbury, Richard Akers, Felicity Hay, Disco, Peter Harbison, Peter Clinch, Jeff Crispin, Cynthia Treadwell, Anagama Kiln, Debbie Lord, Sue Quinlan, Hedder Gabler, , Christine Hateley, Marilyn Chestnut, Geoff Crispen, Petrus Spronk, Peter Ashman, Country Heat, Bruce Widdop, Andrew Seary, Len Bauska, Christopher Pendlebury, Doug Wright, Frank Hurley, Peter Tulloch. Liz Blizzard .9) 1991 - Goldilocks and the Three Bears, Yvonne James, Doll's House, Liz Blizzard, Peter Blizzard, Elizabeth Tanner, Amanda Davies, Kimba Jeffries, Black Comedy and Public Eye, Peter Pilven, Macbeth, Richard Sutherland, Bruce Widdop, The Little Prince, The Would-be Gentleman, The Crucible, Warren Muschialli, Janet Dale, .10) 1992 - Deanne Clapton, Anthony Marsh, Alice in Wonderland, Bruce Widdop, The Beard, Fiona Bennett, Warren Muschialli, Orphans, Peter Blizzard, Red Riding Hood, Circus In a Suitcase, Frank Zappla, The Twelfth Night, Peta Brady, Street Angels, Lawrence Price, Donna Brunt, Jessi Watson, Too Much Punch for Judy, Miranda Crellin, Lyle Quick, Trevor Harris, Howard Tostivan, John Daykin, Barry Breen, The Paradise, Hansel and Gretel, Sandra Moon, Rosalind Lawson, Jason Wasley, The Paradise and The Passion, Simon Buckle, Sam Trinder, Doug Wright, .11) 1998 - Barnum, Skins, Marqui De Sade, Kangaroo Pie, Comedy of Errors, Manhatten, Nicholas Nickleby, Great White Way, Peer Gynt, Boys from Syracuse, Cancerto, Miranda Crellin, Ron McLeod, Alexandra Meerbach, Nathan Firmin, Chris Dickins, Christine Ward, Judith Roberts, Tim Arundell, Dom Phelan, Paul Thomas, Rose Tonkovic, Jon Peck, Andrew Page, Luke Doxey .12) 1999 - Pirates of Penzance, Phil Horwood, Adrian Barnes, Dennis Olsen, Kate Gorman, Nathan Firmin, Margaret Whitlam, Nadine Collins, Liz Gutt, Sara Brett, Kate McLennan, Dom Phelon, Midsummer Night's Dream, Bruce Widdop, Nadia Andary, Amy Maiden, Sing For Your Supper, Tania Burn, Under Milkwood, A month of Sundays, Matt Heyward, Dane Carpenter, Peter Tulloch, phil Crompton, Tim Haymes, David Haymes, Jenny Haymes, Barry Wemyss, Heather Kent, Monty Farag, Sancho de Silva, Jose da Costa, Cameron Sweatman, Ways and Means, Matt Heywood, Rebecca McGuiness, Michelle Pitcher, Peter Tullochperforming arts, visual arts, ballarat academy of performing arts, peter tulloch, bapa -
 Flagstaff Hill Maritime Museum and Village
Flagstaff Hill Maritime Museum and VillageAnimal specimen - Whale bone, Undetermined
Prior to carrying out a detailed condition report of the cetacean skeletons, it is useful to have an understanding of the materials we are likely to encounter, in terms of structure and chemistry. This entry invites you to join in learning about the composition of whale bone and oil. Whale bone (Cetacean) bone is comprised of a composite structure of both an inorganic matrix of mainly hydroxylapatite (a calcium phosphate mineral), providing strength and rigidity, as well as an organic protein ‘scaffolding’ of mainly collagen, facilitating growth and repair (O’Connor 2008, CCI 2010). Collagen is also the structural protein component in cartilage between the whale vertebrae and attached to the fins of both the Killer Whale and the Dolphin. Relative proportions in the bone composition (affecting density), are linked with the feeding habits and mechanical stresses typically endured by bones of particular whale types. A Sperm Whale (Physeter macrocephalus Linnaeus, 1758) skeleton (toothed) thus has a higher mineral value (~67%) than a Fin Whale (Balaenoptera physalus Linnaeus, 1758) (baleen) (~60%) (Turner Walker 2012). The internal structure of bone can be divided into compact and cancellous bone. In whales, load-bearing structures such as mandibles and upper limb bones (e.g. humerus, sternum) are largely composed of compact bone (Turner Walker 2012). This consists of lamella concentrically deposited around the longitudinal axis and is permeated by fluid carrying channels (O’Connor 2008). Cancellous (spongy) bone, with a highly porous angular network of trabeculae, is less stiff and thus found in whale ribs and vertebrae (Turner Walker 2012). Whale oil Whales not only carry a thick layer of fat (blubber) in the soft tissue of their body for heat insulation and as a food store while they are alive, but also hold large oil (lipid) reserves in their porous bones. Following maceration of the whale skeleton after death to remove the soft tissue, the bones retain a high lipid content (Higgs et. al 2010). Particularly bones with a spongy (porous) structure have a high capacity to hold oil-rich marrow. Comparative data of various whale species suggests the skull, particularly the cranium and mandible bones are particularly oil rich. Along the vertebral column, the lipid content is reduced, particularly in the thoracic vertebrae (~10-25%), yet greatly increases from the lumbar to the caudal vertebrae (~40-55%). The chest area (scapula, sternum and ribs) show a mid-range lipid content (~15-30%), with vertically orientated ribs being more heavily soaked lower down (Turner Walker 2012, Higgs et. al 2010). Whale oil is largely composed of triglycerides (molecules of fatty acids attached to a glycerol molecule). In Arctic whales a higher proportion of unsaturated, versus saturated fatty acids make up the lipid. Unsaturated fatty acids (with double or triple carbon bonds causing chain kinks, preventing close packing (solidifying) of molecules), are more likely to be liquid (oil), versus solid (fat) at room temperature (Smith and March 2007). Objects Made From the Whaling Industry We all know that men set forth in sailing ships and risked their lives to harpoon whales on the open seas throughout the 1800s. And while Moby Dick and other tales have made whaling stories immortal, people today generally don't appreciate that the whalers were part of a well-organized industry. The ships that set out from ports in New England roamed as far as the Pacific in hunt of specific species of whales. Adventure may have been the draw for some whalers, but for the captains who owned whaling ships, and the investors which financed voyages, there was a considerable monetary payoff. The gigantic carcasses of whales were chopped and boiled down and turned into products such as the fine oil needed to lubricate increasing advanced machine tools. And beyond the oil derived from whales, even their bones, in an era before the invention of plastic, was used to make a wide variety of consumer goods. In short, whales were a valuable natural resource the same as wood, minerals, or petroleum we now pump from the ground. Oil From Whale’s Blubber Oil was the main product sought from whales, and it was used to lubricate machinery and to provide illumination by burning it in lamps. When a whale was killed, it was towed to the ship and its blubber, the thick insulating fat under its skin, would be peeled and cut from its carcass in a process known as “flensing.” The blubber was minced into chunks and boiled in large vats on board the whaling ship, producing oil. The oil taken from whale blubber was packaged in casks and transported back to the whaling ship’s home port (such as New Bedford, Massachusetts, the busiest American whaling port in the mid-1800s). From the ports it would be sold and transported across the country and would find its way into a huge variety of products. Whale oil, in addition to be used for lubrication and illumination, was also used to manufacture soaps, paint, and varnish. Whale oil was also utilized in some processes used to manufacture textiles and rope. Spermaceti, a Highly Regarded Oil A peculiar oil found in the head of the sperm whale, spermaceti, was highly prized. The oil was waxy, and was commonly used in making candles. In fact, candles made of spermaceti were considered the best in the world, producing a bright clear flame without an excess of smoke. Spermaceti was also used, distilled in liquid form, as an oil to fuel lamps. The main American whaling port, New Bedford, Massachusetts, was thus known as "The City That Lit the World." When John Adams was the ambassador to Great Britain before serving as president he recorded in his diary a conversation about spermaceti he had with the British Prime Minister William Pitt. Adams, keen to promote the New England whaling industry, was trying to convince the British to import spermaceti sold by American whalers, which the British could use to fuel street lamps. The British were not interested. In his diary, Adams wrote that he told Pitt, “the fat of the spermaceti whale gives the clearest and most beautiful flame of any substance that is known in nature, and we are surprised you prefer darkness, and consequent robberies, burglaries, and murders in your streets to receiving as a remittance our spermaceti oil.” Despite the failed sales pitch John Adams made in the late 1700s, the American whaling industry boomed in the early to mid-1800s. And spermaceti was a major component of that success. Spermaceti could be refined into a lubricant that was ideal for precision machinery. The machine tools that made the growth of industry possible in the United States were lubricated, and essentially made possible, by oil derived from spermaceti. Baleen, or "Whalebone" The bones and teeth of various species of whales were used in a number of products, many of them common implements in a 19th century household. Whales are said to have produced “the plastic of the 1800s.” The "bone" of the whale which was most commonly used wasn’t technically a bone, it was baleen, a hard material arrayed in large plates, like gigantic combs, in the mouths of some species of whales. The purpose of the baleen is to act as a sieve, catching tiny organisms in sea water, which the whale consumes as food. As baleen was tough yet flexible, it could be used in a number of practical applications. And it became commonly known as "whalebone." Perhaps the most common use of whalebone was in the manufacture of corsets, which fashionable ladies in the 1800s wore to compress their waistlines. One typical corset advertisement from the 1800s proudly proclaims, “Real Whalebone Only Used.” Whalebone was also used for collar stays, buggy whips, and toys. Its remarkable flexibility even caused it to be used as the springs in early typewriters. The comparison to plastic is apt. Think of common items which today might be made of plastic, and it's likely that similar items in the 1800s would have been made of whalebone. Baleen whales do not have teeth. But the teeth of other whales, such as the sperm whale, would be used as ivory in such products as chess pieces, piano keys, or the handles of walking sticks. Pieces of scrimshaw, or carved whale's teeth, would probably be the best remembered use of whale's teeth. However, the carved teeth were created to pass the time on whaling voyages and were never a mass production item. Their relative rarity, of course, is why genuine pieces of 19th century scrimshaw are considered to be valuable collectibles today. Reference: McNamara, Robert. "Objects Made From the Whaling Industry." ThoughtCo, Jul. 31, 2021, thoughtco.com/products-produced-from-whales-1774070.Whale bone was an important commodity, used in corsets, collar stays, buggy whips, and toys.Whale bone in two pieces. Advanced stage of calcification as indicated by deep pitting. Off white to grey.None.flagstaff hill, warrnambool, shipwrecked-coast, flagstaff-hill, flagstaff-hill-maritime-museum, maritime-museum, shipwreck-coast, flagstaff-hill-maritime-village, whale bones, whale skeleton, whales, whale bone, corsets, toys, whips -
 Flagstaff Hill Maritime Museum and Village
Flagstaff Hill Maritime Museum and VillageAnimal specimen - Whale bone, Undetermined
Prior to carrying out a detailed condition report of the cetacean skeletons, it is useful to have an understanding of the materials we are likely to encounter, in terms of structure and chemistry. This entry invites you to join in learning about the composition of whale bone and oil. Whale bone (Cetacean) bone is comprised of a composite structure of both an inorganic matrix of mainly hydroxylapatite (a calcium phosphate mineral), providing strength and rigidity, as well as an organic protein ‘scaffolding’ of mainly collagen, facilitating growth and repair (O’Connor 2008, CCI 2010). Collagen is also the structural protein component in cartilage between the whale vertebrae and attached to the fins of both the Killer Whale and the Dolphin. Relative proportions in the bone composition (affecting density), are linked with the feeding habits and mechanical stresses typically endured by bones of particular whale types. A Sperm Whale (Physeter macrocephalus Linnaeus, 1758) skeleton (toothed) thus has a higher mineral value (~67%) than a Fin Whale (Balaenoptera physalus Linnaeus, 1758) (baleen) (~60%) (Turner Walker 2012). The internal structure of bone can be divided into compact and cancellous bone. In whales, load-bearing structures such as mandibles and upper limb bones (e.g. humerus, sternum) are largely composed of compact bone (Turner Walker 2012). This consists of lamella concentrically deposited around the longitudinal axis and is permeated by fluid carrying channels (O’Connor 2008). Cancellous (spongy) bone, with a highly porous angular network of trabeculae, is less stiff and thus found in whale ribs and vertebrae (Turner Walker 2012). Whale oil Whales not only carry a thick layer of fat (blubber) in the soft tissue of their body for heat insulation and as a food store while they are alive, but also hold large oil (lipid) reserves in their porous bones. Following maceration of the whale skeleton after death to remove the soft tissue, the bones retain a high lipid content (Higgs et. al 2010). Particularly bones with a spongy (porous) structure have a high capacity to hold oil-rich marrow. Comparative data of various whale species suggests the skull, particularly the cranium and mandible bones are particularly oil rich. Along the vertebral column, the lipid content is reduced, particularly in the thoracic vertebrae (~10-25%), yet greatly increases from the lumbar to the caudal vertebrae (~40-55%). The chest area (scapula, sternum and ribs) show a mid-range lipid content (~15-30%), with vertically orientated ribs being more heavily soaked lower down (Turner Walker 2012, Higgs et. al 2010). Whale oil is largely composed of triglycerides (molecules of fatty acids attached to a glycerol molecule). In Arctic whales a higher proportion of unsaturated, versus saturated fatty acids make up the lipid. Unsaturated fatty acids (with double or triple carbon bonds causing chain kinks, preventing close packing (solidifying) of molecules), are more likely to be liquid (oil), versus solid (fat) at room temperature (Smith and March 2007). Objects Made From the Whaling Industry We all know that men set forth in sailing ships and risked their lives to harpoon whales on the open seas throughout the 1800s. And while Moby Dick and other tales have made whaling stories immortal, people today generally don't appreciate that the whalers were part of a well-organized industry. The ships that set out from ports in New England roamed as far as the Pacific in hunt of specific species of whales. Adventure may have been the draw for some whalers, but for the captains who owned whaling ships, and the investors which financed voyages, there was a considerable monetary payoff. The gigantic carcasses of whales were chopped and boiled down and turned into products such as the fine oil needed to lubricate increasing advanced machine tools. And beyond the oil derived from whales, even their bones, in an era before the invention of plastic, was used to make a wide variety of consumer goods. In short, whales were a valuable natural resource the same as wood, minerals, or petroleum we now pump from the ground. Oil From Whale’s Blubber Oil was the main product sought from whales, and it was used to lubricate machinery and to provide illumination by burning it in lamps. When a whale was killed, it was towed to the ship and its blubber, the thick insulating fat under its skin, would be peeled and cut from its carcass in a process known as “flensing.” The blubber was minced into chunks and boiled in large vats on board the whaling ship, producing oil. The oil taken from whale blubber was packaged in casks and transported back to the whaling ship’s home port (such as New Bedford, Massachusetts, the busiest American whaling port in the mid-1800s). From the ports it would be sold and transported across the country and would find its way into a huge variety of products. Whale oil, in addition to be used for lubrication and illumination, was also used to manufacture soaps, paint, and varnish. Whale oil was also utilized in some processes used to manufacture textiles and rope. Spermaceti, a Highly Regarded Oil A peculiar oil found in the head of the sperm whale, spermaceti, was highly prized. The oil was waxy, and was commonly used in making candles. In fact, candles made of spermaceti were considered the best in the world, producing a bright clear flame without an excess of smoke. Spermaceti was also used, distilled in liquid form, as an oil to fuel lamps. The main American whaling port, New Bedford, Massachusetts, was thus known as "The City That Lit the World." When John Adams was the ambassador to Great Britain before serving as president he recorded in his diary a conversation about spermaceti he had with the British Prime Minister William Pitt. Adams, keen to promote the New England whaling industry, was trying to convince the British to import spermaceti sold by American whalers, which the British could use to fuel street lamps. The British were not interested. In his diary, Adams wrote that he told Pitt, “the fat of the spermaceti whale gives the clearest and most beautiful flame of any substance that is known in nature, and we are surprised you prefer darkness, and consequent robberies, burglaries, and murders in your streets to receiving as a remittance our spermaceti oil.” Despite the failed sales pitch John Adams made in the late 1700s, the American whaling industry boomed in the early to mid-1800s. And spermaceti was a major component of that success. Spermaceti could be refined into a lubricant that was ideal for precision machinery. The machine tools that made the growth of industry possible in the United States were lubricated, and essentially made possible, by oil derived from spermaceti. Baleen, or "Whalebone" The bones and teeth of various species of whales were used in a number of products, many of them common implements in a 19th century household. Whales are said to have produced “the plastic of the 1800s.” The "bone" of the whale which was most commonly used wasn’t technically a bone, it was baleen, a hard material arrayed in large plates, like gigantic combs, in the mouths of some species of whales. The purpose of the baleen is to act as a sieve, catching tiny organisms in sea water, which the whale consumes as food. As baleen was tough yet flexible, it could be used in a number of practical applications. And it became commonly known as "whalebone." Perhaps the most common use of whalebone was in the manufacture of corsets, which fashionable ladies in the 1800s wore to compress their waistlines. One typical corset advertisement from the 1800s proudly proclaims, “Real Whalebone Only Used.” Whalebone was also used for collar stays, buggy whips, and toys. Its remarkable flexibility even caused it to be used as the springs in early typewriters. The comparison to plastic is apt. Think of common items which today might be made of plastic, and it's likely that similar items in the 1800s would have been made of whalebone. Baleen whales do not have teeth. But the teeth of other whales, such as the sperm whale, would be used as ivory in such products as chess pieces, piano keys, or the handles of walking sticks. Pieces of scrimshaw, or carved whale's teeth, would probably be the best remembered use of whale's teeth. However, the carved teeth were created to pass the time on whaling voyages and were never a mass production item. Their relative rarity, of course, is why genuine pieces of 19th century scrimshaw are considered to be valuable collectibles today. Reference: McNamara, Robert. "Objects Made From the Whaling Industry." ThoughtCo, Jul. 31, 2021, thoughtco.com/products-produced-from-whales-1774070.Whale bone was an important commodity, used in corsets, collar stays, buggy whips, and toys.Whale bone piece. Advanced stage of calcification as indicated by deep pitting. Off white to grey.None.flagstaff hill, warrnambool, shipwrecked-coast, flagstaff-hill, flagstaff-hill-maritime-museum, maritime-museum, shipwreck-coast, flagstaff-hill-maritime-village, whales, whale bone, corsets, toys, whips -
 Flagstaff Hill Maritime Museum and Village
Flagstaff Hill Maritime Museum and VillageAnimal specimen - Whale bone, Undetermined
Prior to carrying out a detailed condition report of the cetacean skeletons, it is useful to have an understanding of the materials we are likely to encounter, in terms of structure and chemistry. This entry invites you to join in learning about the composition of whale bone and oil. Whale bone (Cetacean) bone is comprised of a composite structure of both an inorganic matrix of mainly hydroxylapatite (a calcium phosphate mineral), providing strength and rigidity, as well as an organic protein ‘scaffolding’ of mainly collagen, facilitating growth and repair (O’Connor 2008, CCI 2010). Collagen is also the structural protein component in cartilage between the whale vertebrae and attached to the fins of both the Killer Whale and the Dolphin. Relative proportions in the bone composition (affecting density), are linked with the feeding habits and mechanical stresses typically endured by bones of particular whale types. A Sperm Whale (Physeter macrocephalus Linnaeus, 1758) skeleton (toothed) thus has a higher mineral value (~67%) than a Fin Whale (Balaenoptera physalus Linnaeus, 1758) (baleen) (~60%) (Turner Walker 2012). The internal structure of bone can be divided into compact and cancellous bone. In whales, load-bearing structures such as mandibles and upper limb bones (e.g. humerus, sternum) are largely composed of compact bone (Turner Walker 2012). This consists of lamella concentrically deposited around the longitudinal axis and is permeated by fluid carrying channels (O’Connor 2008). Cancellous (spongy) bone, with a highly porous angular network of trabeculae, is less stiff and thus found in whale ribs and vertebrae (Turner Walker 2012). Whale oil Whales not only carry a thick layer of fat (blubber) in the soft tissue of their body for heat insulation and as a food store while they are alive, but also hold large oil (lipid) reserves in their porous bones. Following maceration of the whale skeleton after death to remove the soft tissue, the bones retain a high lipid content (Higgs et. al 2010). Particularly bones with a spongy (porous) structure have a high capacity to hold oil-rich marrow. Comparative data of various whale species suggests the skull, particularly the cranium and mandible bones are particularly oil rich. Along the vertebral column, the lipid content is reduced, particularly in the thoracic vertebrae (~10-25%), yet greatly increases from the lumbar to the caudal vertebrae (~40-55%). The chest area (scapula, sternum and ribs) show a mid-range lipid content (~15-30%), with vertically orientated ribs being more heavily soaked lower down (Turner Walker 2012, Higgs et. al 2010). Whale oil is largely composed of triglycerides (molecules of fatty acids attached to a glycerol molecule). In Arctic whales a higher proportion of unsaturated, versus saturated fatty acids make up the lipid. Unsaturated fatty acids (with double or triple carbon bonds causing chain kinks, preventing close packing (solidifying) of molecules), are more likely to be liquid (oil), versus solid (fat) at room temperature (Smith and March 2007). Objects Made From the Whaling Industry We all know that men set forth in sailing ships and risked their lives to harpoon whales on the open seas throughout the 1800s. And while Moby Dick and other tales have made whaling stories immortal, people today generally don't appreciate that the whalers were part of a well-organized industry. The ships that set out from ports in New England roamed as far as the Pacific in hunt of specific species of whales. Adventure may have been the draw for some whalers, but for the captains who owned whaling ships, and the investors which financed voyages, there was a considerable monetary payoff. The gigantic carcasses of whales were chopped and boiled down and turned into products such as the fine oil needed to lubricate increasing advanced machine tools. And beyond the oil derived from whales, even their bones, in an era before the invention of plastic, was used to make a wide variety of consumer goods. In short, whales were a valuable natural resource the same as wood, minerals, or petroleum we now pump from the ground. Oil From Whale’s Blubber Oil was the main product sought from whales, and it was used to lubricate machinery and to provide illumination by burning it in lamps. When a whale was killed, it was towed to the ship and its blubber, the thick insulating fat under its skin, would be peeled and cut from its carcass in a process known as “flensing.” The blubber was minced into chunks and boiled in large vats on board the whaling ship, producing oil. The oil taken from whale blubber was packaged in casks and transported back to the whaling ship’s home port (such as New Bedford, Massachusetts, the busiest American whaling port in the mid-1800s). From the ports it would be sold and transported across the country and would find its way into a huge variety of products. Whale oil, in addition to be used for lubrication and illumination, was also used to manufacture soaps, paint, and varnish. Whale oil was also utilized in some processes used to manufacture textiles and rope. Spermaceti, a Highly Regarded Oil A peculiar oil found in the head of the sperm whale, spermaceti, was highly prized. The oil was waxy, and was commonly used in making candles. In fact, candles made of spermaceti were considered the best in the world, producing a bright clear flame without an excess of smoke. Spermaceti was also used, distilled in liquid form, as an oil to fuel lamps. The main American whaling port, New Bedford, Massachusetts, was thus known as "The City That Lit the World." When John Adams was the ambassador to Great Britain before serving as president he recorded in his diary a conversation about spermaceti he had with the British Prime Minister William Pitt. Adams, keen to promote the New England whaling industry, was trying to convince the British to import spermaceti sold by American whalers, which the British could use to fuel street lamps. The British were not interested. In his diary, Adams wrote that he told Pitt, “the fat of the spermaceti whale gives the clearest and most beautiful flame of any substance that is known in nature, and we are surprised you prefer darkness, and consequent robberies, burglaries, and murders in your streets to receiving as a remittance our spermaceti oil.” Despite the failed sales pitch John Adams made in the late 1700s, the American whaling industry boomed in the early to mid-1800s. And spermaceti was a major component of that success. Spermaceti could be refined into a lubricant that was ideal for precision machinery. The machine tools that made the growth of industry possible in the United States were lubricated, and essentially made possible, by oil derived from spermaceti. Baleen, or "Whalebone" The bones and teeth of various species of whales were used in a number of products, many of them common implements in a 19th century household. Whales are said to have produced “the plastic of the 1800s.” The "bone" of the whale which was most commonly used wasn’t technically a bone, it was baleen, a hard material arrayed in large plates, like gigantic combs, in the mouths of some species of whales. The purpose of the baleen is to act as a sieve, catching tiny organisms in sea water, which the whale consumes as food. As baleen was tough yet flexible, it could be used in a number of practical applications. And it became commonly known as "whalebone." Perhaps the most common use of whalebone was in the manufacture of corsets, which fashionable ladies in the 1800s wore to compress their waistlines. One typical corset advertisement from the 1800s proudly proclaims, “Real Whalebone Only Used.” Whalebone was also used for collar stays, buggy whips, and toys. Its remarkable flexibility even caused it to be used as the springs in early typewriters. The comparison to plastic is apt. Think of common items which today might be made of plastic, and it's likely that similar items in the 1800s would have been made of whalebone. Baleen whales do not have teeth. But the teeth of other whales, such as the sperm whale, would be used as ivory in such products as chess pieces, piano keys, or the handles of walking sticks. Pieces of scrimshaw, or carved whale's teeth, would probably be the best remembered use of whale's teeth. However, the carved teeth were created to pass the time on whaling voyages and were never a mass production item. Their relative rarity, of course, is why genuine pieces of 19th century scrimshaw are considered to be valuable collectibles today. Reference: McNamara, Robert. "Objects Made From the Whaling Industry." ThoughtCo, Jul. 31, 2021, thoughtco.com/products-produced-from-whales-1774070. Whale bone was an important commodity, used in corsets, collar stays, buggy whips, and toys.Whale bone vertebrae. Advanced stage of calcification as indicated by deep pitting. Off white to grey.None.flagstaff hill, warrnambool, shipwrecked-coast, flagstaff-hill, flagstaff-hill-maritime-museum, maritime-museum, shipwreck-coast, flagstaff-hill-maritime-village, whales, whale bone, corsets, toys, whips -
 Flagstaff Hill Maritime Museum and Village
Flagstaff Hill Maritime Museum and VillageAnimal specimen - Whale bone, Undetermined
Prior to carrying out a detailed condition report of the cetacean skeletons, it is useful to have an understanding of the materials we are likely to encounter, in terms of structure and chemistry. This entry invites you to join in learning about the composition of whale bone and oil. Whale bone (Cetacean) bone is comprised of a composite structure of both an inorganic matrix of mainly hydroxylapatite (a calcium phosphate mineral), providing strength and rigidity, as well as an organic protein ‘scaffolding’ of mainly collagen, facilitating growth and repair (O’Connor 2008, CCI 2010). Collagen is also the structural protein component in cartilage between the whale vertebrae and attached to the fins of both the Killer Whale and the Dolphin. Relative proportions in the bone composition (affecting density), are linked with the feeding habits and mechanical stresses typically endured by bones of particular whale types. A Sperm Whale (Physeter macrocephalus Linnaeus, 1758) skeleton (toothed) thus has a higher mineral value (~67%) than a Fin Whale (Balaenoptera physalus Linnaeus, 1758) (baleen) (~60%) (Turner Walker 2012). The internal structure of bone can be divided into compact and cancellous bone. In whales, load-bearing structures such as mandibles and upper limb bones (e.g. humerus, sternum) are largely composed of compact bone (Turner Walker 2012). This consists of lamella concentrically deposited around the longitudinal axis and is permeated by fluid carrying channels (O’Connor 2008). Cancellous (spongy) bone, with a highly porous angular network of trabeculae, is less stiff and thus found in whale ribs and vertebrae (Turner Walker 2012). Whale oil Whales not only carry a thick layer of fat (blubber) in the soft tissue of their body for heat insulation and as a food store while they are alive, but also hold large oil (lipid) reserves in their porous bones. Following maceration of the whale skeleton after death to remove the soft tissue, the bones retain a high lipid content (Higgs et. al 2010). Particularly bones with a spongy (porous) structure have a high capacity to hold oil-rich marrow. Comparative data of various whale species suggests the skull, particularly the cranium and mandible bones are particularly oil rich. Along the vertebral column, the lipid content is reduced, particularly in the thoracic vertebrae (~10-25%), yet greatly increases from the lumbar to the caudal vertebrae (~40-55%). The chest area (scapula, sternum and ribs) show a mid-range lipid content (~15-30%), with vertically orientated ribs being more heavily soaked lower down (Turner Walker 2012, Higgs et. al 2010). Whale oil is largely composed of triglycerides (molecules of fatty acids attached to a glycerol molecule). In Arctic whales a higher proportion of unsaturated, versus saturated fatty acids make up the lipid. Unsaturated fatty acids (with double or triple carbon bonds causing chain kinks, preventing close packing (solidifying) of molecules), are more likely to be liquid (oil), versus solid (fat) at room temperature (Smith and March 2007). Objects Made From the Whaling Industry We all know that men set forth in sailing ships and risked their lives to harpoon whales on the open seas throughout the 1800s. And while Moby Dick and other tales have made whaling stories immortal, people today generally don't appreciate that the whalers were part of a well-organized industry. The ships that set out from ports in New England roamed as far as the Pacific in hunt of specific species of whales. Adventure may have been the draw for some whalers, but for the captains who owned whaling ships, and the investors which financed voyages, there was a considerable monetary payoff. The gigantic carcasses of whales were chopped and boiled down and turned into products such as the fine oil needed to lubricate increasing advanced machine tools. And beyond the oil derived from whales, even their bones, in an era before the invention of plastic, was used to make a wide variety of consumer goods. In short, whales were a valuable natural resource the same as wood, minerals, or petroleum we now pump from the ground. Oil From Whale’s Blubber Oil was the main product sought from whales, and it was used to lubricate machinery and to provide illumination by burning it in lamps. When a whale was killed, it was towed to the ship and its blubber, the thick insulating fat under its skin, would be peeled and cut from its carcass in a process known as “flensing.” The blubber was minced into chunks and boiled in large vats on board the whaling ship, producing oil. The oil taken from whale blubber was packaged in casks and transported back to the whaling ship’s home port (such as New Bedford, Massachusetts, the busiest American whaling port in the mid-1800s). From the ports it would be sold and transported across the country and would find its way into a huge variety of products. Whale oil, in addition to be used for lubrication and illumination, was also used to manufacture soaps, paint, and varnish. Whale oil was also utilized in some processes used to manufacture textiles and rope. Spermaceti, a Highly Regarded Oil A peculiar oil found in the head of the sperm whale, spermaceti, was highly prized. The oil was waxy, and was commonly used in making candles. In fact, candles made of spermaceti were considered the best in the world, producing a bright clear flame without an excess of smoke. Spermaceti was also used, distilled in liquid form, as an oil to fuel lamps. The main American whaling port, New Bedford, Massachusetts, was thus known as "The City That Lit the World." When John Adams was the ambassador to Great Britain before serving as president he recorded in his diary a conversation about spermaceti he had with the British Prime Minister William Pitt. Adams, keen to promote the New England whaling industry, was trying to convince the British to import spermaceti sold by American whalers, which the British could use to fuel street lamps. The British were not interested. In his diary, Adams wrote that he told Pitt, “the fat of the spermaceti whale gives the clearest and most beautiful flame of any substance that is known in nature, and we are surprised you prefer darkness, and consequent robberies, burglaries, and murders in your streets to receiving as a remittance our spermaceti oil.” Despite the failed sales pitch John Adams made in the late 1700s, the American whaling industry boomed in the early to mid-1800s. And spermaceti was a major component of that success. Spermaceti could be refined into a lubricant that was ideal for precision machinery. The machine tools that made the growth of industry possible in the United States were lubricated, and essentially made possible, by oil derived from spermaceti. Baleen, or "Whalebone" The bones and teeth of various species of whales were used in a number of products, many of them common implements in a 19th century household. Whales are said to have produced “the plastic of the 1800s.” The "bone" of the whale which was most commonly used wasn’t technically a bone, it was baleen, a hard material arrayed in large plates, like gigantic combs, in the mouths of some species of whales. The purpose of the baleen is to act as a sieve, catching tiny organisms in sea water, which the whale consumes as food. As baleen was tough yet flexible, it could be used in a number of practical applications. And it became commonly known as "whalebone." Perhaps the most common use of whalebone was in the manufacture of corsets, which fashionable ladies in the 1800s wore to compress their waistlines. One typical corset advertisement from the 1800s proudly proclaims, “Real Whalebone Only Used.” Whalebone was also used for collar stays, buggy whips, and toys. Its remarkable flexibility even caused it to be used as the springs in early typewriters. The comparison to plastic is apt. Think of common items which today might be made of plastic, and it's likely that similar items in the 1800s would have been made of whalebone. Baleen whales do not have teeth. But the teeth of other whales, such as the sperm whale, would be used as ivory in such products as chess pieces, piano keys, or the handles of walking sticks. Pieces of scrimshaw, or carved whale's teeth, would probably be the best remembered use of whale's teeth. However, the carved teeth were created to pass the time on whaling voyages and were never a mass production item. Their relative rarity, of course, is why genuine pieces of 19th century scrimshaw are considered to be valuable collectibles today. Reference: McNamara, Robert. "Objects Made From the Whaling Industry." ThoughtCo, Jul. 31, 2021, thoughtco.com/products-produced-from-whales-1774070.Whale bone was an important commodity, used in corsets, collar stays, buggy whips, and toys.Whale bone piece. Advanced stage of calcification as indicated by deep pitting. Off white to grey.None.flagstaff hill, warrnambool, shipwrecked-coast, flagstaff-hill, flagstaff-hill-maritime-museum, maritime-museum, shipwreck-coast, flagstaff-hill-maritime-village, whales, whale bone, corsets, toys, whips -
 Flagstaff Hill Maritime Museum and Village
Flagstaff Hill Maritime Museum and VillageAnimal specimen - Whale bone, Undetermined
Prior to carrying out a detailed condition report of the cetacean skeletons, it is useful to have an understanding of the materials we are likely to encounter, in terms of structure and chemistry. This entry invites you to join in learning about the composition of whale bone and oil. Whale bone (Cetacean) bone is comprised of a composite structure of both an inorganic matrix of mainly hydroxylapatite (a calcium phosphate mineral), providing strength and rigidity, as well as an organic protein ‘scaffolding’ of mainly collagen, facilitating growth and repair (O’Connor 2008, CCI 2010). Collagen is also the structural protein component in cartilage between the whale vertebrae and attached to the fins of both the Killer Whale and the Dolphin. Relative proportions in the bone composition (affecting density), are linked with the feeding habits and mechanical stresses typically endured by bones of particular whale types. A Sperm Whale (Physeter macrocephalus Linnaeus, 1758) skeleton (toothed) thus has a higher mineral value (~67%) than a Fin Whale (Balaenoptera physalus Linnaeus, 1758) (baleen) (~60%) (Turner Walker 2012). The internal structure of bone can be divided into compact and cancellous bone. In whales, load-bearing structures such as mandibles and upper limb bones (e.g. humerus, sternum) are largely composed of compact bone (Turner Walker 2012). This consists of lamella concentrically deposited around the longitudinal axis and is permeated by fluid carrying channels (O’Connor 2008). Cancellous (spongy) bone, with a highly porous angular network of trabeculae, is less stiff and thus found in whale ribs and vertebrae (Turner Walker 2012). Whale oil Whales not only carry a thick layer of fat (blubber) in the soft tissue of their body for heat insulation and as a food store while they are alive, but also hold large oil (lipid) reserves in their porous bones. Following maceration of the whale skeleton after death to remove the soft tissue, the bones retain a high lipid content (Higgs et. al 2010). Particularly bones with a spongy (porous) structure have a high capacity to hold oil-rich marrow. Comparative data of various whale species suggests the skull, particularly the cranium and mandible bones are particularly oil rich. Along the vertebral column, the lipid content is reduced, particularly in the thoracic vertebrae (~10-25%), yet greatly increases from the lumbar to the caudal vertebrae (~40-55%). The chest area (scapula, sternum and ribs) show a mid-range lipid content (~15-30%), with vertically orientated ribs being more heavily soaked lower down (Turner Walker 2012, Higgs et. al 2010). Whale oil is largely composed of triglycerides (molecules of fatty acids attached to a glycerol molecule). In Arctic whales a higher proportion of unsaturated, versus saturated fatty acids make up the lipid. Unsaturated fatty acids (with double or triple carbon bonds causing chain kinks, preventing close packing (solidifying) of molecules), are more likely to be liquid (oil), versus solid (fat) at room temperature (Smith and March 2007). Objects Made From the Whaling Industry We all know that men set forth in sailing ships and risked their lives to harpoon whales on the open seas throughout the 1800s. And while Moby Dick and other tales have made whaling stories immortal, people today generally don't appreciate that the whalers were part of a well-organized industry. The ships that set out from ports in New England roamed as far as the Pacific in hunt of specific species of whales. Adventure may have been the draw for some whalers, but for the captains who owned whaling ships, and the investors which financed voyages, there was a considerable monetary payoff. The gigantic carcasses of whales were chopped and boiled down and turned into products such as the fine oil needed to lubricate increasing advanced machine tools. And beyond the oil derived from whales, even their bones, in an era before the invention of plastic, was used to make a wide variety of consumer goods. In short, whales were a valuable natural resource the same as wood, minerals, or petroleum we now pump from the ground. Oil From Whale’s Blubber Oil was the main product sought from whales, and it was used to lubricate machinery and to provide illumination by burning it in lamps. When a whale was killed, it was towed to the ship and its blubber, the thick insulating fat under its skin, would be peeled and cut from its carcass in a process known as “flensing.” The blubber was minced into chunks and boiled in large vats on board the whaling ship, producing oil. The oil taken from whale blubber was packaged in casks and transported back to the whaling ship’s home port (such as New Bedford, Massachusetts, the busiest American whaling port in the mid-1800s). From the ports it would be sold and transported across the country and would find its way into a huge variety of products. Whale oil, in addition to be used for lubrication and illumination, was also used to manufacture soaps, paint, and varnish. Whale oil was also utilized in some processes used to manufacture textiles and rope. Spermaceti, a Highly Regarded Oil A peculiar oil found in the head of the sperm whale, spermaceti, was highly prized. The oil was waxy, and was commonly used in making candles. In fact, candles made of spermaceti were considered the best in the world, producing a bright clear flame without an excess of smoke. Spermaceti was also used, distilled in liquid form, as an oil to fuel lamps. The main American whaling port, New Bedford, Massachusetts, was thus known as "The City That Lit the World." When John Adams was the ambassador to Great Britain before serving as president he recorded in his diary a conversation about spermaceti he had with the British Prime Minister William Pitt. Adams, keen to promote the New England whaling industry, was trying to convince the British to import spermaceti sold by American whalers, which the British could use to fuel street lamps. The British were not interested. In his diary, Adams wrote that he told Pitt, “the fat of the spermaceti whale gives the clearest and most beautiful flame of any substance that is known in nature, and we are surprised you prefer darkness, and consequent robberies, burglaries, and murders in your streets to receiving as a remittance our spermaceti oil.” Despite the failed sales pitch John Adams made in the late 1700s, the American whaling industry boomed in the early to mid-1800s. And spermaceti was a major component of that success. Spermaceti could be refined into a lubricant that was ideal for precision machinery. The machine tools that made the growth of industry possible in the United States were lubricated, and essentially made possible, by oil derived from spermaceti. Baleen, or "Whalebone" The bones and teeth of various species of whales were used in a number of products, many of them common implements in a 19th century household. Whales are said to have produced “the plastic of the 1800s.” The "bone" of the whale which was most commonly used wasn’t technically a bone, it was baleen, a hard material arrayed in large plates, like gigantic combs, in the mouths of some species of whales. The purpose of the baleen is to act as a sieve, catching tiny organisms in sea water, which the whale consumes as food. As baleen was tough yet flexible, it could be used in a number of practical applications. And it became commonly known as "whalebone." Perhaps the most common use of whalebone was in the manufacture of corsets, which fashionable ladies in the 1800s wore to compress their waistlines. One typical corset advertisement from the 1800s proudly proclaims, “Real Whalebone Only Used.” Whalebone was also used for collar stays, buggy whips, and toys. Its remarkable flexibility even caused it to be used as the springs in early typewriters. The comparison to plastic is apt. Think of common items which today might be made of plastic, and it's likely that similar items in the 1800s would have been made of whalebone. Baleen whales do not have teeth. But the teeth of other whales, such as the sperm whale, would be used as ivory in such products as chess pieces, piano keys, or the handles of walking sticks. Pieces of scrimshaw, or carved whale's teeth, would probably be the best remembered use of whale's teeth. However, the carved teeth were created to pass the time on whaling voyages and were never a mass production item. Their relative rarity, of course, is why genuine pieces of 19th century scrimshaw are considered to be valuable collectibles today. Reference: McNamara, Robert. "Objects Made From the Whaling Industry." ThoughtCo, Jul. 31, 2021, thoughtco.com/products-produced-from-whales-1774070.Whale bone was an important commodity, used in corsets, collar stays, buggy whips, and toys.Whale bone piece. Advanced stage of calcification as indicated by deep pitting. Off white to grey.None.flagstaff hill, warrnambool, shipwrecked-coast, flagstaff-hill, flagstaff-hill-maritime-museum, maritime-museum, shipwreck-coast, flagstaff-hill-maritime-village, whales, whale bone, corsets, toys, whips -
 Flagstaff Hill Maritime Museum and Village
Flagstaff Hill Maritime Museum and VillageAnimal specimen - Whale Rib Bone, Undetermined
Prior to carrying out a detailed condition report of the cetacean skeletons, it is useful to have an understanding of the materials we are likely to encounter, in terms of structure and chemistry. This entry invites you to join in learning about the composition of whale bone and oil. Whale bone (Cetacean) bone is comprised of a composite structure of both an inorganic matrix of mainly hydroxylapatite (a calcium phosphate mineral), providing strength and rigidity, as well as an organic protein ‘scaffolding’ of mainly collagen, facilitating growth and repair (O’Connor 2008, CCI 2010). Collagen is also the structural protein component in cartilage between the whale vertebrae and attached to the fins of both the Killer Whale and the Dolphin. Relative proportions in the bone composition (affecting density), are linked with the feeding habits and mechanical stresses typically endured by bones of particular whale types. A Sperm Whale (Physeter macrocephalus Linnaeus, 1758) skeleton (toothed) thus has a higher mineral value (~67%) than a Fin Whale (Balaenoptera physalus Linnaeus, 1758) (baleen) (~60%) (Turner Walker 2012). The internal structure of bone can be divided into compact and cancellous bone. In whales, load-bearing structures such as mandibles and upper limb bones (e.g. humerus, sternum) are largely composed of compact bone (Turner Walker 2012). This consists of lamella concentrically deposited around the longitudinal axis and is permeated by fluid carrying channels (O’Connor 2008). Cancellous (spongy) bone, with a highly porous angular network of trabeculae, is less stiff and thus found in whale ribs and vertebrae (Turner Walker 2012). Whale oil Whales not only carry a thick layer of fat (blubber) in the soft tissue of their body for heat insulation and as a food store while they are alive, but also hold large oil (lipid) reserves in their porous bones. Following maceration of the whale skeleton after death to remove the soft tissue, the bones retain a high lipid content (Higgs et. al 2010). Particularly bones with a spongy (porous) structure have a high capacity to hold oil-rich marrow. Comparative data of various whale species suggests the skull, particularly the cranium and mandible bones are particularly oil rich. Along the vertebral column, the lipid content is reduced, particularly in the thoracic vertebrae (~10-25%), yet greatly increases from the lumbar to the caudal vertebrae (~40-55%). The chest area (scapula, sternum and ribs) show a mid-range lipid content (~15-30%), with vertically orientated ribs being more heavily soaked lower down (Turner Walker 2012, Higgs et. al 2010). Whale oil is largely composed of triglycerides (molecules of fatty acids attached to a glycerol molecule). In Arctic whales a higher proportion of unsaturated, versus saturated fatty acids make up the lipid. Unsaturated fatty acids (with double or triple carbon bonds causing chain kinks, preventing close packing (solidifying) of molecules), are more likely to be liquid (oil), versus solid (fat) at room temperature (Smith and March 2007). Objects Made From the Whaling Industry We all know that men set forth in sailing ships and risked their lives to harpoon whales on the open seas throughout the 1800s. And while Moby Dick and other tales have made whaling stories immortal, people today generally don't appreciate that the whalers were part of a well-organized industry. The ships that set out from ports in New England roamed as far as the Pacific in hunt of specific species of whales. Adventure may have been the draw for some whalers, but for the captains who owned whaling ships, and the investors which financed voyages, there was a considerable monetary payoff. The gigantic carcasses of whales were chopped and boiled down and turned into products such as the fine oil needed to lubricate increasing advanced machine tools. And beyond the oil derived from whales, even their bones, in an era before the invention of plastic, was used to make a wide variety of consumer goods. In short, whales were a valuable natural resource the same as wood, minerals, or petroleum we now pump from the ground. Oil From Whale’s Blubber Oil was the main product sought from whales, and it was used to lubricate machinery and to provide illumination by burning it in lamps. When a whale was killed, it was towed to the ship and its blubber, the thick insulating fat under its skin, would be peeled and cut from its carcass in a process known as “flensing.” The blubber was minced into chunks and boiled in large vats on board the whaling ship, producing oil. The oil taken from whale blubber was packaged in casks and transported back to the whaling ship’s home port (such as New Bedford, Massachusetts, the busiest American whaling port in the mid-1800s). From the ports it would be sold and transported across the country and would find its way into a huge variety of products. Whale oil, in addition to be used for lubrication and illumination, was also used to manufacture soaps, paint, and varnish. Whale oil was also utilized in some processes used to manufacture textiles and rope. Spermaceti, a Highly Regarded Oil A peculiar oil found in the head of the sperm whale, spermaceti, was highly prized. The oil was waxy, and was commonly used in making candles. In fact, candles made of spermaceti were considered the best in the world, producing a bright clear flame without an excess of smoke. Spermaceti was also used, distilled in liquid form, as an oil to fuel lamps. The main American whaling port, New Bedford, Massachusetts, was thus known as "The City That Lit the World." When John Adams was the ambassador to Great Britain before serving as president he recorded in his diary a conversation about spermaceti he had with the British Prime Minister William Pitt. Adams, keen to promote the New England whaling industry, was trying to convince the British to import spermaceti sold by American whalers, which the British could use to fuel street lamps. The British were not interested. In his diary, Adams wrote that he told Pitt, “the fat of the spermaceti whale gives the clearest and most beautiful flame of any substance that is known in nature, and we are surprised you prefer darkness, and consequent robberies, burglaries, and murders in your streets to receiving as a remittance our spermaceti oil.” Despite the failed sales pitch John Adams made in the late 1700s, the American whaling industry boomed in the early to mid-1800s. And spermaceti was a major component of that success. Spermaceti could be refined into a lubricant that was ideal for precision machinery. The machine tools that made the growth of industry possible in the United States were lubricated, and essentially made possible, by oil derived from spermaceti. Baleen, or "Whalebone" The bones and teeth of various species of whales were used in a number of products, many of them common implements in a 19th century household. Whales are said to have produced “the plastic of the 1800s.” The "bone" of the whale which was most commonly used wasn’t technically a bone, it was baleen, a hard material arrayed in large plates, like gigantic combs, in the mouths of some species of whales. The purpose of the baleen is to act as a sieve, catching tiny organisms in sea water, which the whale consumes as food. As baleen was tough yet flexible, it could be used in a number of practical applications. And it became commonly known as "whalebone." Perhaps the most common use of whalebone was in the manufacture of corsets, which fashionable ladies in the 1800s wore to compress their waistlines. One typical corset advertisement from the 1800s proudly proclaims, “Real Whalebone Only Used.” Whalebone was also used for collar stays, buggy whips, and toys. Its remarkable flexibility even caused it to be used as the springs in early typewriters. The comparison to plastic is apt. Think of common items which today might be made of plastic, and it's likely that similar items in the 1800s would have been made of whalebone. Baleen whales do not have teeth. But the teeth of other whales, such as the sperm whale, would be used as ivory in such products as chess pieces, piano keys, or the handles of walking sticks. Pieces of scrimshaw, or carved whale's teeth, would probably be the best remembered use of whale's teeth. However, the carved teeth were created to pass the time on whaling voyages and were never a mass production item. Their relative rarity, of course, is why genuine pieces of 19th century scrimshaw are considered to be valuable collectibles today. Reference: McNamara, Robert. "Objects Made From the Whaling Industry." ThoughtCo, Jul. 31, 2021, thoughtco.com/products-produced-from-whales-1774070.Whale bone during the 17th, 18th, 19th and early 20th centuries was an important industry providing an important commodity. Whales from these times provided everything from lighting & machine oils to using the animal's bones for use in corsets, collar stays, buggy whips, and many other everyday items then in use.Whale rib bone with advanced stage of calcification as indicated by brittleness. None.warrnambool, flagstaff-hill, flagstaff-hill-maritime-museum, maritime-museum, shipwreck-coast, flagstaff-hill-maritime-village, whale bones, whale skeleton, whales, whale bone, corsets, toys, whips, whaleling industry, maritime fishing, whalebone -
 Flagstaff Hill Maritime Museum and Village
Flagstaff Hill Maritime Museum and VillageAnimal specimen - Whale bone, Undetermined
Prior to carrying out a detailed condition report of the cetacean skeletons, it is useful to have an understanding of the materials we are likely to encounter, in terms of structure and chemistry. This entry invites you to join in learning about the composition of whale bone and oil. Whale bone (Cetacean) bone is comprised of a composite structure of both an inorganic matrix of mainly hydroxylapatite (a calcium phosphate mineral), providing strength and rigidity, as well as an organic protein ‘scaffolding’ of mainly collagen, facilitating growth and repair (O’Connor 2008, CCI 2010). Collagen is also the structural protein component in cartilage between the whale vertebrae and attached to the fins of both the Killer Whale and the Dolphin. Relative proportions in the bone composition (affecting density), are linked with the feeding habits and mechanical stresses typically endured by bones of particular whale types. A Sperm Whale (Physeter macrocephalus Linnaeus, 1758) skeleton (toothed) thus has a higher mineral value (~67%) than a Fin Whale (Balaenoptera physalus Linnaeus, 1758) (baleen) (~60%) (Turner Walker 2012). The internal structure of bone can be divided into compact and cancellous bone. In whales, load-bearing structures such as mandibles and upper limb bones (e.g. humerus, sternum) are largely composed of compact bone (Turner Walker 2012). This consists of lamella concentrically deposited around the longitudinal axis and is permeated by fluid carrying channels (O’Connor 2008). Cancellous (spongy) bone, with a highly porous angular network of trabeculae, is less stiff and thus found in whale ribs and vertebrae (Turner Walker 2012). Whale oil Whales not only carry a thick layer of fat (blubber) in the soft tissue of their body for heat insulation and as a food store while they are alive, but also hold large oil (lipid) reserves in their porous bones. Following maceration of the whale skeleton after death to remove the soft tissue, the bones retain a high lipid content (Higgs et. al 2010). Particularly bones with a spongy (porous) structure have a high capacity to hold oil-rich marrow. Comparative data of various whale species suggests the skull, particularly the cranium and mandible bones are particularly oil rich. Along the vertebral column, the lipid content is reduced, particularly in the thoracic vertebrae (~10-25%), yet greatly increases from the lumbar to the caudal vertebrae (~40-55%). The chest area (scapula, sternum and ribs) show a mid-range lipid content (~15-30%), with vertically orientated ribs being more heavily soaked lower down (Turner Walker 2012, Higgs et. al 2010). Whale oil is largely composed of triglycerides (molecules of fatty acids attached to a glycerol molecule). In Arctic whales a higher proportion of unsaturated, versus saturated fatty acids make up the lipid. Unsaturated fatty acids (with double or triple carbon bonds causing chain kinks, preventing close packing (solidifying) of molecules), are more likely to be liquid (oil), versus solid (fat) at room temperature (Smith and March 2007). Objects Made From the Whaling Industry We all know that men set forth in sailing ships and risked their lives to harpoon whales on the open seas throughout the 1800s. And while Moby Dick and other tales have made whaling stories immortal, people today generally don't appreciate that the whalers were part of a well-organized industry. The ships that set out from ports in New England roamed as far as the Pacific in hunt of specific species of whales. Adventure may have been the draw for some whalers, but for the captains who owned whaling ships, and the investors which financed voyages, there was a considerable monetary payoff. The gigantic carcasses of whales were chopped and boiled down and turned into products such as the fine oil needed to lubricate increasing advanced machine tools. And beyond the oil derived from whales, even their bones, in an era before the invention of plastic, was used to make a wide variety of consumer goods. In short, whales were a valuable natural resource the same as wood, minerals, or petroleum we now pump from the ground. Oil From Whale’s Blubber Oil was the main product sought from whales, and it was used to lubricate machinery and to provide illumination by burning it in lamps. When a whale was killed, it was towed to the ship and its blubber, the thick insulating fat under its skin, would be peeled and cut from its carcass in a process known as “flensing.” The blubber was minced into chunks and boiled in large vats on board the whaling ship, producing oil. The oil taken from whale blubber was packaged in casks and transported back to the whaling ship’s home port (such as New Bedford, Massachusetts, the busiest American whaling port in the mid-1800s). From the ports it would be sold and transported across the country and would find its way into a huge variety of products. Whale oil, in addition to be used for lubrication and illumination, was also used to manufacture soaps, paint, and varnish. Whale oil was also utilized in some processes used to manufacture textiles and rope. Spermaceti, a Highly Regarded Oil A peculiar oil found in the head of the sperm whale, spermaceti, was highly prized. The oil was waxy, and was commonly used in making candles. In fact, candles made of spermaceti were considered the best in the world, producing a bright clear flame without an excess of smoke. Spermaceti was also used, distilled in liquid form, as an oil to fuel lamps. The main American whaling port, New Bedford, Massachusetts, was thus known as "The City That Lit the World." When John Adams was the ambassador to Great Britain before serving as president he recorded in his diary a conversation about spermaceti he had with the British Prime Minister William Pitt. Adams, keen to promote the New England whaling industry, was trying to convince the British to import spermaceti sold by American whalers, which the British could use to fuel street lamps. The British were not interested. In his diary, Adams wrote that he told Pitt, “the fat of the spermaceti whale gives the clearest and most beautiful flame of any substance that is known in nature, and we are surprised you prefer darkness, and consequent robberies, burglaries, and murders in your streets to receiving as a remittance our spermaceti oil.” Despite the failed sales pitch John Adams made in the late 1700s, the American whaling industry boomed in the early to mid-1800s. And spermaceti was a major component of that success. Spermaceti could be refined into a lubricant that was ideal for precision machinery. The machine tools that made the growth of industry possible in the United States were lubricated, and essentially made possible, by oil derived from spermaceti. Baleen, or "Whalebone" The bones and teeth of various species of whales were used in a number of products, many of them common implements in a 19th century household. Whales are said to have produced “the plastic of the 1800s.” The "bone" of the whale which was most commonly used wasn’t technically a bone, it was baleen, a hard material arrayed in large plates, like gigantic combs, in the mouths of some species of whales. The purpose of the baleen is to act as a sieve, catching tiny organisms in sea water, which the whale consumes as food. As baleen was tough yet flexible, it could be used in a number of practical applications. And it became commonly known as "whalebone." Perhaps the most common use of whalebone was in the manufacture of corsets, which fashionable ladies in the 1800s wore to compress their waistlines. One typical corset advertisement from the 1800s proudly proclaims, “Real Whalebone Only Used.” Whalebone was also used for collar stays, buggy whips, and toys. Its remarkable flexibility even caused it to be used as the springs in early typewriters. The comparison to plastic is apt. Think of common items which today might be made of plastic, and it's likely that similar items in the 1800s would have been made of whalebone. Baleen whales do not have teeth. But the teeth of other whales, such as the sperm whale, would be used as ivory in such products as chess pieces, piano keys, or the handles of walking sticks. Pieces of scrimshaw, or carved whale's teeth, would probably be the best remembered use of whale's teeth. However, the carved teeth were created to pass the time on whaling voyages and were never a mass production item. Their relative rarity, of course, is why genuine pieces of 19th century scrimshaw are considered to be valuable collectibles today. Reference: McNamara, Robert. "Objects Made From the Whaling Industry." ThoughtCo, Jul. 31, 2021, thoughtco.com/products-produced-from-whales-1774070.Whale bone was an important commodity, used in corsets, collar stays, buggy whips, and toys.Whale bone vertebrae. Advanced stage of calcification as indicated by deep pitting. Off white to grey.Noneflagstaff hill, warrnambool, shipwrecked-coast, flagstaff-hill, flagstaff-hill-maritime-museum, maritime-museum, shipwreck-coast, flagstaff-hill-maritime-village, whales, whale bone, corsets, toys, whips, whalebone -
 Flagstaff Hill Maritime Museum and Village
Flagstaff Hill Maritime Museum and VillageAnimal specimen - Whale Vertebrae, Undetermined
Prior to carrying out a detailed condition report of the cetacean skeletons, it is useful to have an understanding of the materials we are likely to encounter, in terms of structure and chemistry. This entry invites you to join in learning about the composition of whale bone and oil. Whale bone (Cetacean) bone is comprised of a composite structure of both an inorganic matrix of mainly hydroxylapatite (a calcium phosphate mineral), providing strength and rigidity, as well as an organic protein ‘scaffolding’ of mainly collagen, facilitating growth and repair (O’Connor 2008, CCI 2010). Collagen is also the structural protein component in cartilage between the whale vertebrae and attached to the fins of both the Killer Whale and the Dolphin. Relative proportions in the bone composition (affecting density), are linked with the feeding habits and mechanical stresses typically endured by bones of particular whale types. A Sperm Whale (Physeter macrocephalus Linnaeus, 1758) skeleton (toothed) thus has a higher mineral value (~67%) than a Fin Whale (Balaenoptera physalus Linnaeus, 1758) (baleen) (~60%) (Turner Walker 2012). The internal structure of bone can be divided into compact and cancellous bone. In whales, load-bearing structures such as mandibles and upper limb bones (e.g. humerus, sternum) are largely composed of compact bone (Turner Walker 2012). This consists of lamella concentrically deposited around the longitudinal axis and is permeated by fluid carrying channels (O’Connor 2008). Cancellous (spongy) bone, with a highly porous angular network of trabeculae, is less stiff and thus found in whale ribs and vertebrae (Turner Walker 2012). Whale oil Whales not only carry a thick layer of fat (blubber) in the soft tissue of their body for heat insulation and as a food store while they are alive, but also hold large oil (lipid) reserves in their porous bones. Following maceration of the whale skeleton after death to remove the soft tissue, the bones retain a high lipid content (Higgs et. al 2010). Particularly bones with a spongy (porous) structure have a high capacity to hold oil-rich marrow. Comparative data of various whale species suggests the skull, particularly the cranium and mandible bones are particularly oil rich. Along the vertebral column, the lipid content is reduced, particularly in the thoracic vertebrae (~10-25%), yet greatly increases from the lumbar to the caudal vertebrae (~40-55%). The chest area (scapula, sternum and ribs) show a mid-range lipid content (~15-30%), with vertically orientated ribs being more heavily soaked lower down (Turner Walker 2012, Higgs et. al 2010). Whale oil is largely composed of triglycerides (molecules of fatty acids attached to a glycerol molecule). In Arctic whales a higher proportion of unsaturated, versus saturated fatty acids make up the lipid. Unsaturated fatty acids (with double or triple carbon bonds causing chain kinks, preventing close packing (solidifying) of molecules), are more likely to be liquid (oil), versus solid (fat) at room temperature (Smith and March 2007). Objects Made From the Whaling Industry We all know that men set forth in sailing ships and risked their lives to harpoon whales on the open seas throughout the 1800s. And while Moby Dick and other tales have made whaling stories immortal, people today generally don't appreciate that the whalers were part of a well-organized industry. The ships that set out from ports in New England roamed as far as the Pacific in hunt of specific species of whales. Adventure may have been the draw for some whalers, but for the captains who owned whaling ships, and the investors which financed voyages, there was a considerable monetary payoff. The gigantic carcasses of whales were chopped and boiled down and turned into products such as the fine oil needed to lubricate increasing advanced machine tools. And beyond the oil derived from whales, even their bones, in an era before the invention of plastic, was used to make a wide variety of consumer goods. In short, whales were a valuable natural resource the same as wood, minerals, or petroleum we now pump from the ground. Oil From Whale’s Blubber Oil was the main product sought from whales, and it was used to lubricate machinery and to provide illumination by burning it in lamps. When a whale was killed, it was towed to the ship and its blubber, the thick insulating fat under its skin, would be peeled and cut from its carcass in a process known as “flensing.” The blubber was minced into chunks and boiled in large vats on board the whaling ship, producing oil. The oil taken from whale blubber was packaged in casks and transported back to the whaling ship’s home port (such as New Bedford, Massachusetts, the busiest American whaling port in the mid-1800s). From the ports it would be sold and transported across the country and would find its way into a huge variety of products. Whale oil, in addition to be used for lubrication and illumination, was also used to manufacture soaps, paint, and varnish. Whale oil was also utilized in some processes used to manufacture textiles and rope. Spermaceti, a Highly Regarded Oil A peculiar oil found in the head of the sperm whale, spermaceti, was highly prized. The oil was waxy, and was commonly used in making candles. In fact, candles made of spermaceti were considered the best in the world, producing a bright clear flame without an excess of smoke. Spermaceti was also used, distilled in liquid form, as an oil to fuel lamps. The main American whaling port, New Bedford, Massachusetts, was thus known as "The City That Lit the World." When John Adams was the ambassador to Great Britain before serving as president he recorded in his diary a conversation about spermaceti he had with the British Prime Minister William Pitt. Adams, keen to promote the New England whaling industry, was trying to convince the British to import spermaceti sold by American whalers, which the British could use to fuel street lamps. The British were not interested. In his diary, Adams wrote that he told Pitt, “the fat of the spermaceti whale gives the clearest and most beautiful flame of any substance that is known in nature, and we are surprised you prefer darkness, and consequent robberies, burglaries, and murders in your streets to receiving as a remittance our spermaceti oil.” Despite the failed sales pitch John Adams made in the late 1700s, the American whaling industry boomed in the early to mid-1800s. And spermaceti was a major component of that success. Spermaceti could be refined into a lubricant that was ideal for precision machinery. The machine tools that made the growth of industry possible in the United States were lubricated, and essentially made possible, by oil derived from spermaceti. Whalebone The bones and teeth of various species of whales were used in a number of products, many of them common implements in a 19th century household. Whales are said to have produced “the plastic of the 1800s.” The bone of the whale which was most commonly used wasn’t technically a bone, it was baleen, a hard material arrayed in large plates, like gigantic combs, in the mouths of some species of whales. The purpose of the baleen is to act as a sieve, catching tiny organisms in sea water, which the whale consumes as food. As baleen was tough yet flexible, it could be used in a number of practical applications. And it became commonly known as whalebone. Perhaps the most common use of whalebone was in the manufacture of corsets, which fashionable ladies in the 1800s wore to compress their waistlines. One typical corset advertisement from the 1800s proudly proclaims, “Real Whalebone Only Used.” Whalebone was also used for collar stays, buggy whips, and toys. Its remarkable flexibility even caused it to be used as the springs in early typewriters. The comparison to plastic is apt. Think of common items which today might be made of plastic, and it's likely that similar items in the 1800s would have been made of whalebone. Baleen whales do not have teeth. But the teeth of other whales, such as the sperm whale, would be used as ivory in such products as chess pieces, piano keys, or the handles of walking sticks. Pieces of scrimshaw, or carved whale's teeth, would probably be the best remembered use of whale's teeth. However, the carved teeth were created to pass the time on whaling voyages and were never a mass production item. Their relative rarity, of course, is why genuine pieces of 19th century scrimshaw are considered to be valuable collectibles today. Reference: McNamara, Robert. "Objects Made From the Whaling Industry." ThoughtCo, Jul. 31, 2021, thoughtco.com/products-produced-from-whales-1774070.Whale bone during the 17th, 18th, 19th and early 20th centuries was an important industry providing an important commodity. Whales from these times provided everything from lighting & machine oils to using the animal's bones for use in corsets, collar stays, buggy whips, and many other everyday items then in use.Whale bone Vertebrae with advanced stage of calcification as indicated by deep pitting. Off white to grey.None.warrnambool, flagstaff-hill, flagstaff-hill-maritime-museum, maritime-museum, shipwreck-coast, flagstaff-hill-maritime-village, whale bones, whale skeleton, whales, whale bone, corsets, toys, whips, whaleling industry, maritime fishing, whalebone
