Showing 132 items
matching consumables
-
 Eltham District Historical Society Inc
Eltham District Historical Society IncPhotograph - Digital Photograph, Alan King, St Margaret's Anglican Church, Pitt Street, Eltham, 30 January 2008
St Margaret’s Church of England was officially opened on December 12, 1861. It is the oldest intact church building in Eltham. At the time it was known as Christ Church until its consecration in 1871, when it was completely free of debt (£1,700 for the church and parsonage) despite the district’s poverty. This was largely due to the free labour and materials, including locally made bricks donated by local artisans and others. The church is historically significant because it is the oldest church in the former Shire of Eltham and has associations with the philanthropist and founder of Brighton, Henry Dendy (who donated the land on which the church is built), the architect Nathaniel Billing and the prominent local builder, George Stebbing. The church is architecturally and aesthetically significant because it is constructed in the Gothic Revival style with several stained-glass windows of various dates and is also a very early use of polychromatic brickwork in Victoria. Billing was one of the first Melbourne architects to employ polychromatic brickwork and an important early architect. The rear wall was intended to be temporary. A major feature of the design is the large buttresses with long, steeply graded upper faces. The overall design is well proportioned with the surface brick patterns relieving an otherwise austere design. The church is spiritually and socially significant because it has been an important place of worship for the people of Eltham for almost 150 years. The land on which the buildings stand was donated by Henry Dendy. Dendy arrived in Melbourne in 1841 after purchasing in England eight square miles at Brighton under the system of "special surveys". After this land passed out of his hands, Dendy moved about Victoria, visited England, then returned to settle in Eltham where he purchased a flour mill. Dendy chaired the meeting held in 1860 “for the purpose of devising such means as may be expedient for the establishment of a Church of England in the township of Eltham”. He became chairman and treasurer of the church committee. Unlike the establishment of many early churches in Victoria where a vicar was appointed to a parish and later a permanent church was constructed, the population at Eltham initiated action to build a church. The nearest church at that time was at Heidelberg and the Eltham settlement was part of the parish of St Johns Heidelberg. Isolation and the tedious, time consuming journey between Heidelberg and Eltham resulted in the Eltham community taking its own action. The original vicarage (Dendy House) at the rear of the church is also an important part of the cultural significance of this place because it is connected to the church and the development of the Eltham area. Together, the church and the vicarage are aesthetically significant because they form a significant streetscape feature. The mud-brick community hall designed by Robert Marshall was added in 1978. In 2014 the original temporary rear wall was removed as part of a modern extension designed by Architects Atelier Wagner and constructed by Conrad Construction and Management. Covered under Heritage Overlay, Nillumbik Planning Scheme. National Trust of Australia (Victoria) State significance Victorian Heritage Published: Nillumbik Now and Then / Marguerite Marshall 2008; photographs Alan King with Marguerite Marshall.; p67 St Margaret’s Anglican Church in Pitt Street, Eltham, which officially opened on December 12, 1861, is the oldest intact church building in Eltham.1 With the nearby courthouse and police station, it was one of the first permanent community buildings in the district. The church and vicarage are on the Register of the Heritage Council of Victoria and the National Trust of Australia – Victoria. The church is important as an early example of polychrome brickwork by the notable architect Nathaniel Billings. It is also notable for its historic associations with the early settlement of the Shire of Eltham and its connection with Henry Dendy, Brighton’s founder.2 Henry Dendy, who lived in Eltham much longer than at Brighton, chaired the original meeting which planned the church, and he donated the half-acre (0.2ha) site. Dendy had arrived in Melbourne in 1841 after buying eight square miles (20.7sq km) at Brighton while in England. After this land passed out of his hands, he eventually settled in Eltham where he bought a flour mill, west from the corner of Main Road and Pitt Street (then called Brewery Lane). The vicarage was named Dendy House after him. The Eltham settlers were unusual in initiating the establishment of a church. Usually in Victoria a vicar was appointed to a parish and then a permanent church was constructed. But then, the nearest church was at Heidelberg, which was a tedious and time-consuming journey. St Margaret’s builder was a local, George Stebbing, who also constructed the former Methodist, later Uniting, Church at John Street and the Shillinglaw Cottage near Eltham’s Central Park. It is believed the first Anglican Bishop of Melbourne, Bishop Perry, dedicated the church. After the ceremony he joined in the festivities at the nearby pub and a bill was sent to the parish for teas taken there by the bishop with other participants. The first vicar was the Reverend Robert Mackie from 1864 to1866. St Margaret’s Church was originally called Christ Church until its consecration in 1871, when it was completely free of debt (£1700 pounds for the church and parsonage) despite the district’s poverty. This was largely due to the free labour and materials, including local bricks, donated by local artisans and others. St Margaret’s Church is in the Gothic Revival tradition with a buttressed nave, paired lancet windows, porch and bell-cote. It was the first polychromatic brick church in Australia, using softly contrasting coloured brickwork.3 Billing was one of the first architects to introduce polychrome brickwork into Melbourne. His original drawings for St Margaret’s survive in a folio of his architectural work. However the church’s brickwork is more subdued than in his drawings. About half the windows – those in clear glass with gold borders – are original. The stained glass windows were made much later, but the one behind the altar is thought to be the oldest in the Diamond Valley. It was to be temporary until the congregation could afford to extend the church. In the early 1960s the original cedar pews were replaced by blonde timber pews and the originals were sold to restaurants and to private individuals. Eminent local sculptor Matcham Skipper created a crucifix for the church. A major addition was made in 1978, when the weatherboard hall was replaced by a mud-brick hall. Made of local material, it was designed by local architect and a former shire president Robert Marshall. The mud-brick hall reflects the style of building in Eltham of the late 1970s and for which Eltham is well-known. Perhaps because its earthy tones blend with the surrounding environment, the hall sits well with the church building. St Margaret’s membership has included economist and ABC chairman, Richard Downing; political commentator, diplomat and academic, William Macmahon Ball; Eltham civic leader, Charles Wingrove; artist, Peter Glass; and Eltham’s first postmaster, Frederick Falkiner.This collection of almost 130 photos about places and people within the Shire of Nillumbik, an urban and rural municipality in Melbourne's north, contributes to an understanding of the history of the Shire. Published in 2008 immediately prior to the Black Saturday bushfires of February 7, 2009, it documents sites that were impacted, and in some cases destroyed by the fires. It includes photographs taken especially for the publication, creating a unique time capsule representing the Shire in the early 21st century. It remains the most recent comprehenesive publication devoted to the Shire's history connecting local residents to the past. nillumbik now and then (marshall-king) collection, eltham, st margaret's anglican church, st margaret's church, st margarets church hall, christ church -
 Flagstaff Hill Maritime Museum and Village
Flagstaff Hill Maritime Museum and VillageNail, circa 1810
This copper nail, sometimes known as a ‘Dumpy Bolt’ or spike, was salvaged from the hull of the wreck of the “George III”. It dates back to at least 1810. It was found by an abalone diver on the south east coast of Tasmania. The nail would have been used to hold the layers of the ship’s keel frame and the planking together. The nail has been passed from the abalone diver to an interested business man on a trip to the south of Hobart, on again to the business man’s close friend who then donated it to Flagstaff Hill Maritime Village. The metal of nails such as this one, after being in the sea for a long time, become affected by the natural reaction of the sea water, causing it to degenerate and thin, and the stress from the force of the sea over the years alters its shape. Iron nails had been used on ships previously, but they quickly corroded in the salt; ships needed regular, costly and time-consuming maintenance to replace the iron nails. Towards the end of the 18th century the British Navy trialled the use of copper nails, finding them to be very successful. Merchant ships began to adopt this process in the early 19th century, although it made ship building very expensive and was more often used for ships such as the “George III” that sailed on long voyages. The three masted sailing ship “George III” was a convict transport ship built in Deptford, England, in 1810. On 14th December 1834 she left Woolwich, England, bound for Hobart Town, Van Diemen’s Land (Tasmania), under Captain William Hall Moxey. She was carrying 220 male convicts plus crew, guards and their families, totalling 294 persons (another 2 were during the voyage). Amongst the cargo were military stores including several copper drums of gun powder. On 27th January 1835 the “George III” was near the Equator, about half way into her journey. A fire broke out and the gun powder was in danger of explosion, threatening the whole ship. Two convicts braved the heat and smoke, entered the store and seized the gun powder drums, suffering burns for their efforts but saving a probable disaster. The fire destroyed some of the provisions and food was scarce. Many became ill with scurvy and some died during the journey. Nearing the end of their journey on 10th April 1835 the “George III” was headed through the D'Entrecasteaux Channel, south east Tasmania, between the mainland and Bruny Island. She was sailing in the moonlit night to hasten her arrival in port due to the great number of sick on board. She struck uncharted rocks, known only to the local whalers, between Actaeon Reef and Southport Lagoon and within hours began to break up. The ship’s boats were used to first rescue the women and children. Firearms were used to help quell the panic of the convicts below decks and some were killed by the shots. Many convicts, including the sick, were drowned. In all, 133 lives were lost including 5 of the crew, guards and their families. It was the third worst shipping disaster in Tasmanian waters. A monument in honour of the prisoners who perished in the “George III” has been erected, noting the date of the wreck as “Friday 10th April 1835.” (NOTE: there are a few differences between sources regarding dates of the shipwreck, some saying March and others April 1835. There are also differences in the figures of those on board and the number of lives lost.) The copper nail is significant as an example of sailing ship construction; fasteners used in the early 19th century on ships carrying convicts to Australia. The nail is also significant for its association with the ship “George III”. The “George III” is registered on the Australian National Shipwreck Database, ID 7195 as an Historic Shipwreck. She is the third worst shipwreck in Tasmanian waters. She is also associated with Early Australian History and the transportation of convicts to Australia. The incident of the fire on board and the bravery of the convicts in making the gun powder safe is an example of the social character of the people in early Tasmanian colonisation. Copper nail (also called a Dumpy bolt or spike) from the convict ship George III, wrecked in 1835. Nail is long, bent in an ‘L’ shape about 3/5ths along, tapering from both ends to the bend. Both ends are flat and do not taper to a point, nor have a thread. The shorter end has been polished, showing bright copper. There is pitting along the nail and virdigris is evident on the longer, unpolished end. The nail is displayed with the longer section resting on a wooden board between two ‘U’ shaped uprights, the shorter section upright. flagstaff hill, warrnambool, shipwrecked coast, flagstaff hill maritime museum, maritime museum, shipwreck coast, flagstaff hill maritime village, great ocean road, george iii, ship construction, ship nail, 1835 shipwreck, 19th century shipwreck, william moxey, d'entrecasteaux channel, convict transportation, copper nail, dumpy bolt, spike, keel nail -
 Flagstaff Hill Maritime Museum and Village
Flagstaff Hill Maritime Museum and VillagePhotograph - Historical, Warrnambool, C. 1854-1871
This is an image of the Warrnambool Lighthouse Complex on Middle Island in 1854. The Store, Lighthouse Keeper's Quarters, Lighthouse and Flagstaff are in the background. The foreground shows a covered buggy drawn by two horses and a person in attendance, and another wheeled vehicle behind it with a figure nearby. There is a saddled horse to the right with two males in conversation nearby. The ground is soft, perhaps the riverbed or sandy shore. THE LIGHTHOUSE KEEPERS Lighthouse Keepers were responsible for keeping their Lighthouse’s lights shining at night. They kept a lookout for passing vessels and changes in weather. They were expected to clean, polish and maintain the equipment and buildings. They kept regular and detailed records of who was on watch, and the time the light was lit, trimmed and extinguished. They kept a journal about other events that occurred. They keep regular, accurate Meteorological Logs. It was expected that they were competent in Morse code signalling. They would be called to help in times of disasters and shipwrecks and to give official statements about these events. Many Lighthouse Keepers also volunteered as members of the lifeboat crew. The Lady Bay lighthouses were officially classified as small, so the Keepers had the official titles of Senior Assistant Lighthouse Keeper and Assistant Lighthouse Keeper. They were employed by the Public Service and paid rent to live in the Lighthouse Quarters. They were compulsorily retired at the age of 60, with most receiving a superannuation payment. Despite their time-consuming duties, there was time to follow hobbies and crafts such as growing vegetables, playing musical instruments, making models of buildings including lighthouses, and crafting furniture pieces. An example of a keeper’s skills is the carved fire screen made by /assistant Keeper Thomas Hope in the late 19th century and displayed in the Lighthouse Keeper’s cottage at Flagstaff Hill. Both Alexander and Farncombe had served under Senior Keeper Robert Deverell, who was the first and only Senior Lighthouse Keeper at the Middle Island Complex. John Alexander was the Assistant Keeper in the 1850s. Andrew Farncombe was the last Assistant Keeper at Middle Island, serving there with his family from 1864 to 1871. During 1871 and 1872 the Lighthouse Complex was moved to Flagstaff Hill on Merri Street. Farncombe and Deverell then became the first Keepers and occupants of the Lady Bay Lighthouse Complex at Flagstaff Hill. They continued their service together; overall, Deverell served from 1859 to 1885 and Farncombe from 1864 to 1974. WARRNAMBOOL'S LADY BAY LIGHTHOUSE COMPLEX - The original Lighthouse Complex was built on Middle Island in 1858-1859 then transferred stone-by-stone to Flagstaff Hill in 1871. The Complex comprised the Lighthouse, the Lighthouse Keepers’ Quarters and a Privy. The bluestone Keeper’s Quarters was a cottage divided into two compartments, one for the Senior Keeper and his family, the other for the Assistant Keeper and his family. The bluestone Store was divided into three; a store, a workshop, and an oil store (or office). The Privy comprised a small building also divided into two separate, back-to-back toilets, one for each Keeper and his family. In the 1970s the Flagstaff Hill Planning Board was set up under the chairmanship of John Lindsay. The Board was to make recommendations to the Warrnambool City Council regarding the use of the buildings and the rest of the Crown Land on the site. The Flagstaff Hill Maritime Village opened in 1975 and began renovating the Cottage in stages, during which time evidence of a 1920s fire was found in the eastern section of the cottage. Additions of a porch on the west and a washroom on the east were made in the 1980s. The western part of the building is now a Shipwreck Museum and the east has returned to a late 19th-century Lighthouse Keeper’s cottage and includes the screen made by Assistant Lighthouse Keeper Thomas Hope in the late 19th century. Hope served two periods of time at the Lighthouse. This photograph is significant as a visual record of the original Warrnambool Lighthouse Complex on Middle Island, the origin of what is now the Lady Bay Lighthouse Complex. The photograph is significant for its connection to the Complex, which is now listed on the Victorian Heritage Register, H1520, for being of historical, scientific (technological) and architectural significance to the State of Victoria. The Complex is significant as an example of early colonial development. The photograph is significant for its connection with the important navigational function of the Lighthouses, a function still being performed to this day. The photograph is also significant as it shows an example of buildings organised by the Public Works Department in Victoria in the mid-to-late 19th century. The structures tare still stand strong. Photograph of horses, a buggy and three gentlemen in the foreground and the background shows a lighthouse and accompanying buildings. Printed in black and white. (Another two horse-drawn vehicles are partially visible). The subject is the Lighthouse Complex on Middle Island, Warrnambool, dated between 1854 and 1871.An inscription is handwritten in black pen on the back of the mounting board."The lighthouse and accompanying buildings were / established on Middle Island in 1854, as this / picture shows. In 1871 they were moved to their / present site on Flagstaff Hill."flagstaff hill, flagstaff hill maritime museum, flagstaff hill maritime village, warrnambool, maritime museum, shipwreck coast, lighthouse keeper's cottage, lighthouse residence, lighthouse, chart room, quarters, privy, middle island, beach lighthouse, obelisk, lighthouse complex, lady bay complex, warrnambool port, warrnambool harbour, lady bay, keepers, lighthouse keeper, upper lighthouse, lower lighthouse, assistant keeper, ports and harbours, cottage, meteorological record, 1854, 1871 -
 Flagstaff Hill Maritime Museum and Village
Flagstaff Hill Maritime Museum and VillageContainer - Ink Bottle and Pen, Caldwell’s Ink Factory, Early 20th century
This shaped ink bottle made by Caldwell's is called a 'boat ink bottle'. It was shaped especially to hold a nib pen when the pen was not in use. The design of the bottle is sometimes called a ‘cottage’ or ‘boat’ shape. The Caldwell’s handmade glass ink bottle was mouth-blown into a two-piece mould, a method often used in the mid-to-late 19th century. The glass blower burst the bottle off the end of his blowpipe with a tool, leaving an uneven mouth and sharp edge on the bottle, which was usually filed. The bottle was then filled with ink and sealed with a cork. More expensive bottles would have a lip added, which was more time-consuming and costly to produce. The capacity for a bottle such as this was about 3 ½ oz (ounces) equal to about 100 ml. Pen and ink have been in use for handwriting since about the seventh century. A quill pen made from a bird’s feather was used up until around the mid-19th century. In the 1850s a steel point nib for the dip pen was invented and could be manufactured on machines in large quantities. The nis only held a small amount of ink so users had to frequently dip the nib into an ink well for more ink. Handwriting left wet ink on the paper, so the blotting paper was carefully used to absorb the excess ink and prevent smudging. Ink could be purchased as a ready-to-use liquid or in powdered form, which needed to be mixed with water. In the 1880s a successful, portable fountain pen gave smooth-flowing ink and was easy to use. In the mid-20th century, the modern ballpoint pen was readily available and inexpensive, so the fountain pen lost its popularity. However, artisans continue to use nib pens to create beautiful calligraphy. Caldwell’s Ink Co. – F.R. Caldwell established Caldwell’s Ink Company in Australia around 1902. In Victoria, he operated from a factory at Victoria Avenue, Albert Park, until about 1911, then from Yarra Bank Road in South Melbourne. Newspaper offices were appointed as agencies to sell his inks, for example, in 1904 the New Zealand Evening Star sold Caldwell’s Flo-Eesi blue black ink in various bottle sizes, and Murchison Advocate (Victoria) stocked Caldwell’s ink in crimson, green, blue black, violet, and blue. Caldwell’s ink was stated to be “non-corrosive and unaffected by steel pens”. A motto used in advertising in 1904-1908 reads ‘Makes Writing a Pleasure’. Stationers stocked Caldwell’s products and hawkers sold Caldwell’s ink stands from door to door in Sydney in the 1910s and 1920s. In 1911 Caldwell promised cash for returned ink bottles and warned of prosecution for anyone found refilling his bottles. Caldwell’s Ink Stands were given as gifts. The company encouraged all forms of writing with their Australian-made Flo-Eesi writing inks and bottles at their impressive booth in the ‘All Australian Exhibition’ in 1913. It advertised its other products, which included Caldwell’s Gum, Caldwell’s Stencil Ink (copy ink) and Caldwell’s Quicksticker as well as Caldwell’s ‘Zac’ Cough Mixture. Caldwell stated in a 1920 article that his inks were made from a formula that was over a century old, and were scientifically tested and quality controlled. The formula included gallic and tannic acids and high-quality dyes to ensure that they did not fade. They were “free from all injurious chemicals”. The permanent quality of the ink was important for legal reasons, particularly to banks, accountants, commerce, municipal councils and lawyers. The Caldwell’s Ink Company also exported crates of its ink bottles and ink stands overseas. Newspaper advertisements can be found for Caldwell’s Ink Company up until 1934 when the company said they were the Best in the business for 40 years.This pen and ink bottle set is of significance as the bottle has its original cork and retains remnants of ink, which was made from a recipe that at the time was over 100 years old, according to Caldwell.. The handmade, mould blown method of manufacture is representative of a 19th-century handcraft industry that is now been largely replaced by mass production. The bottle and its contents are of state significance for being produced by an early Melbourne industry and exported overseas. The pen and ink set is historically significant as it represents methods of handwritten communication that were still common up until the mid-20th century when fountain pens and modern ballpoint pens became popular and convenient and typewriters were becoming part of standard office equipment.Victorian boat ink bottle; small rectangular clear glass ink bottle with horizontal grooves made in the glass for resting and holding the pen. The set includes one pen and nib with the bottle and cork. The bottle is made by Caldwell's and contains its Flo-Eesi Blue Black Ink brand."Caldwell's Flo-Eesi Blue Black Ink."flagstaff hill, warrnambool, maritime museum, maritime village, great ocean road, shipwreck coast, ink, nib pen, writing ink, writing, copying, banks, lawyers, commerce, student, permanent ink, flo-eesi, blue black ink, stationery, record keeping, handwriting, writing equipment, writing accessory, office supply, cottage bottle, boat bottle, mouth-blown bottle, two-part mould, sheer-lip bottle, burst-lip, cork seal, f r caldwell, caldwell’s ink company, albert park, south melbourne, inkstands, stencil ink, copy ink, quicksticker, zac cough mixture -
 Flagstaff Hill Maritime Museum and Village
Flagstaff Hill Maritime Museum and VillageContainer - Ink Bottles, Caldwell’s Ink Factory, Early 20th century
This crate of bottles may have come from a wholesaler, business, stationer or school. The design of the bottles is sometimes called a ‘cottage’ or ‘boat’ shape. Each of the 70 Caldwell’s handmade glass ink bottles was mouth-blown into a two-piece mould, a method often used in the mid-to-late 19th century. The glass blower burst the bottle off the end of his blowpipe with a tool, leaving an uneven mouth and sharp edge on the bottle, which was usually filed. The bottle was then filled with ink and sealed with a cork. More expensive bottles would have a lip added, which was more time-consuming and costly to produce. The capacity for a bottle such as this was about 3 ½ oz (ounces) equal to about 100 ml. Pen and ink have been in use for handwriting since about the seventh century. A quill pen made from a bird’s feather was used up until around the mid-19th century. In the 1850s a steel point nib for the dip pen was invented and could be manufactured on machines in large quantities. The nis only held a small amount of ink so users had to frequently dip the nib into an ink well for more ink. Handwriting left wet ink on the paper, so the blotting paper was carefully used to absorb the excess ink and prevent smudging. Ink could be purchased as a ready-to-use liquid or in powdered form, which needed to be mixed with water. In the 1880s a successful, portable fountain pen gave smooth-flowing ink and was easy to use. In the mid-20th century, the modern ballpoint pen was readily available and inexpensive, so the fountain pen lost its popularity. However, artisans continue to use nib pens to create beautiful calligraphy. Caldwell’s Ink Co. – F.R. Caldwell established Caldwell’s Ink Company in Australia around 1902. In Victoria, he operated from a factory at Victoria Avenue, Albert Park, until about 1911, then from Yarra Bank Road in South Melbourne. Newspaper offices were appointed as agencies to sell his inks, for example, in 1904 the New Zealand Evening Star sold Caldwell’s Flo-Eesi blue black ink in various bottle sizes, and Murchison Advocate (Victoria) stocked Caldwell’s ink in crimson, green, blue black, violet, and blue. Caldwell’s ink was stated to be “non-corrosive and unaffected by steel pens”. A motto used in advertising in 1904-1908 reads ‘Makes Writing a Pleasure’. Stationers stocked Caldwell’s products and hawkers sold Caldwell’s ink stands from door to door in Sydney in the 1910s and 1920s. In 1911 Caldwell promised cash for returned ink bottles and warned of prosecution for anyone found refilling his bottles. Caldwell’s Ink Stands were given as gifts. The company encouraged all forms of writing with their Australian-made Flo-Eesi writing inks and bottles at their impressive booth in the ‘All Australian Exhibition’ in 1913. It advertised its other products, which included Caldwell’s Gum, Caldwell’s Stencil Ink (copy ink) and Caldwell’s Quicksticker as well as Caldwell’s ‘Zac’ Cough Mixture. Caldwell stated in a 1920 article that his inks were made from a formula that was over a century old, and were scientifically tested and quality controlled. The formula included gallic and tannic acids and high-quality dyes to ensure that they did not fade. They were “free from all injurious chemicals”. The permanent quality of the ink was important for legal reasons, particularly to banks, accountants, commerce, municipal councils and lawyers. The Caldwell’s Ink Company also exported crates of its ink bottles and ink stands overseas. Newspaper advertisements can be found for Caldwell’s Ink Company up until 1934 when the company said they were the Best in the business for 40 years.This large collection of similar ink bottles is of particular significance as the bottles have come from the same source, most have their original corks and some retain their original labels, which is rare. The method of manufacture of these bottles is also representative of a 19th-century handcraft industry that is now been largely replaced by mass production. The bottles and their contents are of state significance for being produced by an early Melbourne industry and exported overseas. This case of ink bottles is historically significant as it represents methods of handwritten communication that were still common up until the mid-20th century when fountain pens and modern ballpoint pens became popular and convenient and typewriters were becoming part of standard office equipment.Ink bottles in a wooden crate; 70 rectangular, hand-blown clear glass ink bottles. They have side seams, uneven thickness, especially at the bases, and rough, burst-off mouths. The shoulders on the long sides have horizontal grooves used for pen rests. The bottles vary; some have labels, some contain remnants of blue-black ink, and many have their original corks. The glass has bubbles and imperfections. The remnants of printed labels are on white paper with a swirly border and black text. The bottles contained Caldwell’s blend of blue black ‘Flo-Eesi’ ink.Printed on label; “CALDWELL FLO-EESI BLUE BLACK INK” “ - - - - “ Printed script signature “F.R. Caldwell”flagstaff hill, warrnambool, maritime village, maritime museum, shipwreck coast, great ocean road, ink, nib pen, writing ink, writing, copying, banks, lawyers, commerce, student, permanent ink, flo-eesi, blue black ink, stationery, record keeping, handwriting, writing equipment, writing accessory, office supply, cottage bottle, boat bottle, mouth-blown bottle, two-part mould, sheer-lip bottle, burst-lip, cork seal, f r caldwell, caldwell’s ink company, albert park, south melbourne, inkstands, stencil ink, copy ink, quicksticker, zac cough mixture -
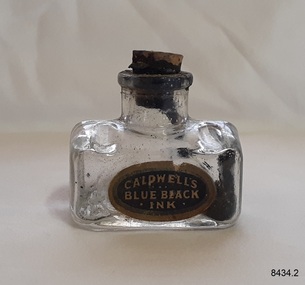 Flagstaff Hill Maritime Museum and Village
Flagstaff Hill Maritime Museum and VillageContainer - Ink Bottle, Caldwell’s Ink Factory, Late 19th to early 20th centuries
This design of the bottle is sometimes called a ‘cottage’ or ‘boat’ shape. The Caldwell’s handmade glass ink bottle was mouth-blown into a three-piece mould, a method often used in the late 19th and early 20th centuries, with the maker's name engraved into the mould section for the base. The glass blower would cut the bottle off the end of his blowpipe with a tool and join a mouth onto the top, rolling the lip. The bottle was then filled with ink and sealed with a cork. This method of manufacture was more time-consuming and costly to produce than those made in a simple two-piece mould and 'cracked' off the blowpipe. The capacity for a bottle such as this was about 3 ½ oz (ounces) equal to about 100 ml. This particular bottle is unusual as it has four sloping indents at the corners of the shoulder, most likely for resting a pen with its nib upwards and the handle resting on a flat surface. Most of the bottles made during this era had horizontal pen rests that were indented into both of the long sides of the shoulder. Pen and ink have been in use for handwriting since about the seventh century. A quill pen made from a bird’s feather was used up until around the mid-19th century. In the 1850s a steel point nib for the dip pen was invented and could be manufactured on machines in large quantities. This only held a small amount of ink so users had to frequently dip the nib into an ink well for more ink. Handwriting left wet ink on the paper, so the blotting paper was carefully used to absorb the excess ink and prevent smudging. Ink could be purchased as a ready-to-use liquid or in powdered form, which needed to be mixed with water. In the 1880s a successful, portable fountain pen gave smooth-flowing ink and was easy to use. In the mid-20th century, the modern ballpoint pen was readily available and inexpensive, so the fountain pen lost its popularity. However, artisans continue to use nib pens to create beautiful calligraphy. Caldwell’s Ink Co. – F.R. Caldwell established Caldwell’s Ink Company in Australia around 1902. In Victoria, he operated from a factory at Victoria Avenue, Albert Park, until about 1911, then from Yarra Bank Road in South Melbourne. Newspaper offices were appointed as agencies to sell his inks, for example, in 1904 the New Zealand Evening Star sold Caldwell’s Flo-Eesi blue black ink in various bottle sizes, and Murchison Advocate (Victoria) stocked Caldwell’s ink in crimson, green, blue black, violet, and blue. Caldwell’s ink was stated to be “non-corrosive and unaffected by steel pens”. A motto used in advertising in 1904-1908 reads ‘Makes Writing a Pleasure’. Stationers stocked Caldwell’s products and hawkers sold Caldwell’s ink stands from door to door in Sydney in the 1910s and 1920s. In 1911 Caldwell promised cash for returned ink bottles and warned of prosecution for anyone found refilling his bottles. Caldwell’s Ink Stands were given as gifts. The company encouraged all forms of writing with their Australian-made Flo-Eesi writing inks and bottles at their impressive booth in the ‘All Australian Exhibition’ in 1913. It advertised its other products, which included Caldwell’s Gum, Caldwell’s Stencil Ink (copy ink) and Caldwell’s Quicksticker as well as Caldwell’s ‘Zac’ Cough Mixture. Caldwell stated in a 1920 article that his inks were made from a formula that was over a century old, and were scientifically tested and quality controlled. The formula included gallic and tannic acids and high-quality dyes to ensure that they did not fade. They were “free from all injurious chemicals”. The permanent quality of the ink was important for legal reasons, particularly to banks, accountants, commerce, municipal councils and lawyers. The Caldwell’s Ink Company also exported crates of its ink bottles and ink stands overseas. Newspaper advertisements can be found for Caldwell’s Ink Company up until 1934 when the company said they were the Best in the business for 40 years.This hand-blown bottle is significant for being the only bottle in our collection with the unusual sloping pen rests on its shoulder. It is also significant for being made in a less common three-piece mould. The method of manufacture is representative of a 19th-century handcraft industry that is now been largely replaced by mass production. The bottle is of state significance for being produced by an early Melbourne industry and exported overseas. This ink bottle is historically significant as it represents methods of handwritten communication that were still common up until the mid-20th century when fountain pens and modern ballpoint pens became popular and convenient and typewriters were becoming part of standard office equipment.Ink bottle; rectangular base, hand-blown clear glass bottle with its own cork. The bottle has side seams from the base to the mouth, an indented base and an applied lip. The corners of the shoulder sides have unusual diagonal grooves that slope down and outwards that may have been used as pen rests. Inside the bottle are remnants of dried blue-black ink. The glass has imperfections and some ripples on the surface. The bottle has an attached oval black label label with gold-brown printed text and border. The base has an embossed inscription. The bottles once contained Caldwell’s blend of blue black ink.Printed on label; “CALDWELL's BLUE BLACK INK” Embossed on the base "CALDWELLS"flagstaff hill, warrnambool, maritime village, maritime museum, shipwreck coast, great ocean road, ink, nib pen, writing ink, writing, copying, banks, lawyers, commerce, student, permanent ink, blue black ink, stationery, record keeping, handwriting, writing equipment, writing accessory, office supply, cottage bottle, boat bottle, mouth-blown bottle, cork seal, f r caldwell, caldwell’s ink company, albert park, south melbourne, inkstands, stencil ink, copy ink, quicksticker, zac cough mixture, three part mould, cauldwells, cauldwell's -
 Flagstaff Hill Maritime Museum and Village
Flagstaff Hill Maritime Museum and VillageDomestic object - Teapot, Unknown
In the 1650s, the newest exciting development had arrived on Britain’s shores, this time it was tea from China. As it was brought back from overseas, tea was incredibly scarce and as such its price was very high; in 1664, the cost of tea was already 40s per pound, although this is not as high as what it would become when taxed in the 18th century. This resulted in only the social elite enjoying a cup of tea, and most commonly tea was enjoyed in coffee houses, and teapots were therefore not yet a household item. As the East India Company imported larger quantities of tea, it became more widely available and a larger section of the British population were able to enjoy it meaning that, by 1669, tea was available nearly everywhere. Likely due to the fact that tea was first enjoyed in coffee houses, the first known teapot resembles a coffee pot, with a tapering cylindrical shape and standing much taller than what we now know as a teapot at 13.5 inches tall. Into the 1680s, these teapots were given a conical cover for the spout that was fixed to the pot via a chain. As Queen Anne took the throne in 1702, teapots had become much more widely used and had formed two common groups. The first style of teapot was the pear shaped style which began to appear in 1705. The pear shaped pot usually had a domed lid and sometimes featured a finial. This form was generally supplied with a heater and stand as well as having a baluster shaped handle on one side. This iteration would disappear by 1725 but does make a reappearance in the 1740s, only this time as an inverted pear shape. The second group was the more spherical, or globular, shape which appeared in 1710. The globular teapot had a flush, hinged lid as well as a narrow moulded rim foot and a straight sided, tapering spout. Both generalised groups of teapots have polygonal examples – that is, teapots that are made up of straight sided segments – but six or seven sided teapots are incredibly rare. There is one known example of a seven sided globular teapot, made by Isaac Ribouleau in 1724. This is so unique because polygonal teapots are much more technically difficult and time consuming to make. Other than the occasional band of engraving round the shoulder of the teapot, they remain quite plain until c.1740 when scrollwork and chased shells begin to be applied for decoration. ‘Chasing’ is the process of decorating the front of a piece of metal by indenting the back, without cutting or engraving. From 1755 until 1770, silver teapots became incredibly uncommon and it is likely that this either reflects a change in drinking habits or changing trends producing a favour for porcelain. This dip in popularity could also be in response to the outrageous taxes placed on tea, up to 119%! In 1765, the Leeds creamware globular teapot seemed to kickstart a resurgence and this, combined with the Commutation Act of 1784 – which reduced tax on tea from 119% to 12.5% – saw teapots return in all their forms. It’s around this time, in 1780, that a form of teapot with a detachable, openwork stand appeared; however, the plain, oval teapot remained the most popular in the 1780s and 90s. In the later years of George III’s tenure on the throne, during the last decade of the 18th century, there was a revival of chasing and embossing teapots with flower and foliage designs. At the turn of the century, the spherical, partly fluted teapot with classical decoration was superseded by a more oblong shaped pot that sat on four spherical feet. This was then changed again when teapots became more melon shaped. It was at this time that the capacity of a teapot greatly increased and the previously wooden or ivory handles were replaced by silver handles with ivory washers for insulation. As Britain entered into the Victorian era, the design quality often suffered as there was a tendency to over-decorate the silver. In the early 19th century, the last major addition to the shape of the teapot, a raised collar was added between the cover and body. Whilst this seems to just be for decoration, there is some speculation that it could also be to prevent overspills. https://www.marklittler.com/silver-teapots-history/ This item shows that silver and silver plated teapots were used for tea making.Plain sliver teapot. Heavy oxidation. Dented.None.flagstaff hill, warrnambool, shipwrecked-coast, flagstaff-hill, flagstaff-hill-maritime-museum, maritime-museum, shipwreck-coast, flagstaff-hill-maritime-village, teapot, silver, siver plate, tea -
 Plutarch Project
Plutarch ProjectFilm screening Log Book, Log Book, Collins late 1950's
Yiannoudes Family Film Memorabilia It is normally a time consuming and difficult task to accurately assess a collection’s significance to the primary criteria, however in the case of the “Yiannoudes Family Film Memorabilia” we have no hesitation of its high significance about its historic, social, rarity, interpretive, cultural and provenance to Australia, including the country side where most of this collection memorabilia visited. From January 1959 and until 1982, “Cosmopolitan Motion Pictures”, owned by Mr Peter Yannoudes (Παναγιώτης Γιαννούδης) and Mr Stathis Raftopoulos (Στάθης Ραφτόπουλος) travelled around Australia to entertain the Greek, Turkish, Indian and Yugoslav speaking population of Australia and provide a significant cinema culture. They travelled as far as Perth in WA, Adelaide in SA, Tasmania, Darwin in Nt, Canberra in ACT and Sydney and NSW. However they found themselves also in places like Berri and Renmark in NSW, where concentrations of migrants lived and thrived during the period. Initially they were travelling by train, carrying all their equipment by hand and placing them in boxes and suitcases. However after 1962 when they acquired their first automobile, travelling became less of a burden, nevertheless cumbersome and laborious. They carried with them initially two portable projectors (second one as a backup) and at times travelled with a third in order to ensure that technology will not be letting them down at the time of film projection. At times the films were projected onto a white sheet of cloth because there was no proper screen to project it on at the venue they were using. Mr P. Yiannoudes has also published a book in October 2010, titled “Greek Cinema Across Australia – Behind the Scenes”. The book was published in two languages, English and in Greek. Details about the launch can be found on the Diasporic Literature Spot website at this address (in the Greek language) http://diasporic.org/ellinika/biblia/greek-films-in-australia/. His book is devoted to those with whom he co-operated in order to bring for the first time Greek language films into Australia. Their names are: Stathis Raftopoulos, Andreas Papadopoulos, Andreas Katopodis, Theodoros Kanellopoulos, Michael Ioannou, Fotis Hatzipavlides, Kostas Vrahnas, Evaggelos Terpenos, Dionysis Lourantos, Dimitris Georgiou, Vasilis Florias and Jim Gragie. All businessmen with the right entrepreneurial spirit to be the first and to make their mark in the making of cultural Australia. Mr P. Yiannoudes a Cypriot by descent born in the town of Vouni, a village in the area of Lemesos. In Lemesos he learned the first few things about cinema which would help him in all his later life. He migrated to Australia in 1956 has been a prominent member of the Greek & Cypriot Communities in Melbourne for many decades. He has been President of the Cypriot Community, President of Federation of Cypriot Communities in Australia (for 18 years), President of SEKA (for 26 years) and highly regarded member of the Greek-Cypriots Diaspora since he also has been Vice-President of the Global Federation of Cypriots of Diaspora for 18 years. Mr P. Yiannoudes is now working on creating a small museum of these pieces in the back of the Westgarth Theatre with the help of the Plutarch Project and …. In this collection numbering hundreds of items, we will try and capture some of the glory that was the Greek film industry in Australia for 23 years between 1959 and 1982. “Cosmopolitan Motion Pictures” also owned a large number of cinemas in Melbourne, the National Theatre in Richmond, the Westgarth Theatre in Northcote (which is still owned by the Yiannoudes family today), Sun Theatre in Yarraville, Kinema in Albert Park, Empire Theatre in Brunswick, Paramount Theatre in Oakleigh, Globe Theatre in Richmond, Galaxy Theatre in Brunswick and the Cosmopolitan Theatre in Brusnwick. At the same time they were hiring other theatres for film projections. They were the Astor Theatre in St. Kilda, Victoria Theatre in Richmond, Sunshine Theatre in Sunshine. Apart from Melbourne they were using the Pantheon Theatre in Adelaide, the Norwood Town Hall in Adelaide, the Shepparton Town Hall in Shepparton, the Premier Theatre in Perth, the Rivoli Theatre in Berri and the Renmark Theatre in Renmark. The number of films shown around Australia were over 1500 in total whilst about 1218 of them were in the Greek language. Other languages shown were in Turkish (about 150 films), Yugoslavian (about 100 films), English, French, German, Swedish, Dutch language films. “Cosmopolitan Motion Pictures” was the first company to bring Swedish and Dutch films to Australia. They also showed Martial Arts films for the first time in Australia in 1975 at the Galaxy Theatre in Melbourne. However one of the most significant pieces that tell the story with places and dates is the Show Logbook. The Show Logbook has a large number of stories to tell. It is still intact and in fair condition after all these years of travelling around Australia. It is categorised with an alphabetic index on the right by film title. Greek, Indian, Turkish and Yugoslav language film titles adorn its pages alongside the place where they were first shown, the towns and cities they visited and the dates for each one. It is an extremely significant part of history of the settlement of migrants in Australia. This Log Book is of Primary Significance to the "Cosmopolitan Motion Pictures" and the Yiannoudes family film memorabilia collection. It has a Historic, Social, Provenance and Rarity significance for the settlement of migrants in Australia and the entertainment industry.This is the Log Book, manually updated and used by "Cosmopolitan Motion Pictures" for films shown in different parts of AustraliaCollins Stock Records Booklogbook, films, shown, cultural, language, greek, australia, γιαννούδης, κατάσταση, yiannoudes -
 Flagstaff Hill Maritime Museum and Village
Flagstaff Hill Maritime Museum and VillageDomestic object - Fork
Naturally, we tend to take commonplace objects for granted, because they have always been there. Yet how many of you actually have thought “hey, where do forks come from?” Well, it takes one trip to China and a 3-year-old laughing at your face because of your desperate attempt to eat with chopsticks to finally appreciate something so ordinary such as a fork. So, where do forks come from? The early history of the fork is obscure. As a kitchen and dining utensil, it is believed to have originated in the Roman Empire, as proved by archaeological evidence. The personal table fork most likely originated in the Eastern Roman (or Byzantine) Empire. Its use spread to what is now the Middle East during the first millennium AD and then spread into Southern Europe during the second millennium. It did not become common in northern Europe until the 18th century and was not common in North America until the 19th century. Carving fork from 1640. Source: Wikipedia/Public Domain Carving Fork from 1640. Source: Wikipedia/Public Domain Some of the earliest known uses of forks with food occurred in Ancient Egypt, where large forks were used as cooking utensils. Bone forks had been found on the burial site of the Bronze Age Qijia culture (2400–1900 BC) as well as later Chinese dynasties’ tombs.The Ancient Greeks used the fork as a serving utensil. Read also: Steven Spielberg to Remake the Classic Musical ‘West Side Story’ In the Roman Empire, bronze and silver forks were used. The use varied according to local customs, social class and the nature of food, but forks of the earlier periods were mostly used as cooking and serving utensils. The personal table fork was most likely invented in the Eastern Roman (Byzantine) Empire, where they were in everyday use by the 4th century (its origin may even go back to Ancient Greece, before the Roman period). Records show that by the 9th century a similar utensil known as a barjyn was in limited use in Persia within some elite circles. By the 10th century, the table fork was in common use throughout the Middle East. Bronze forks made in Persia during the 8th or 9th century.Source: Wikipedia/Public Domain Bronze forks made in Persia during the 8th or 9th century.Source: Wikipedia/Public Domain The first recorded introduction of the fork to Western Europe, as recorded by the theologian and Cardinal Peter Damian, was by Theophano Sklereina the Byzantine wife of Holy Roman Emperor Otto II, who nonchalantly wielded one at an Imperial banquet in 972, astonishing her Western hosts.By the 11th century, the table fork had become increasingly prevalent in the Italian peninsula. It gained a following in Italy before any other Western European region because of historical ties with Byzantium and continued to get popularity due to the increasing presence of pasta in the Italian diet. At first, pasta was consumed using a long wooden spike, but this eventually evolved into three spikes, design better suited to gathering the noodles. In Italy, it became commonplace by the 14th century and was almost universally used by the merchant and upper classes by 1600. It was proper for a guest to arrive with his fork and spoon enclosed in a box called a cadena; this usage was introduced to the French court with Catherine de’ Medici’s entourage. In Portugal, forks were first used at the time of Infanta Beatrice, Duchess of Viseu, King Manuel I of Portugal’s mother around 1450. However, forks were not commonly used in Western Europe until the 16th century when they became part of Italian etiquette. The utensil had also gained some currency in Spain by this time, and its use gradually spread to France. Nevertheless, most of Europe did not adopt the use of the fork until the 18th century. Read also: The 8 Most Famous ‘Functioning Alcoholics’ in History Long after the personal table fork had become commonplace in France, at the supper celebrating the marriage of the Duc de Chartres to Louis XIV’s natural daughter in 1692, the seating was described in the court memoirs of Saint-Simon: “King James having his Queen on his right hand and the King on his left, and each with their cadenas.” In Perrault’s contemporaneous fairy tale of La Belle au bois dormant (1697), each of the fairies invited for the christening is presented with a splendid “fork holder”. The fork’s adoption in northern Europe was slower. Its use was first described in English by Thomas Coryat in a volume of writings on his Italian travels (1611), but for many years it was viewed as an unmanly Italian affectation. Some writers of the Roman Catholic Church expressly disapproved of its use, St. Peter Damian seeing it as “excessive delicacy.” It was not until the 18th century that the fork became commonly used in Great Britain, although some sources say that forks were common in France, England, and Sweden already by the early 17th century. Spaghetti fork By Lady alys - Own work, CC BY-SA 3.0, https://commons.wikimedia.org/w/index.php?curid=6414948 Spaghetti Fork By Lady alys – Own work, CC BY-SA 3.0, The fork did not become popular in North America until near the time of the American Revolution. The curved fork used in most parts of the world today was developed in Germany in the mid 18th century while the standard four-tine design became current in the early 19th century. The fork was important in Germany because they believed that eating with the fingers was rude and disrespectful. The fork led to family dinners and sit-down meals, which are important features of German culture. https://www.thevintagenews.com/2016/08/31/priority-fork-came-italy-european-country-pasta/?chrome=1Serving fork, two prongs, with a shaped wooden handle. Badly rusted.None.flagstaff hill, warrnambool, shipwrecked-coast, flagstaff-hill, flagstaff-hill-maritime-museum, maritime-museum, shipwreck-coast, flagstaff-hill-maritime-village, food, meat, carving -
 Coal Creek Community Park & Museum
Coal Creek Community Park & MuseumBottle, glass, c. 1885
150 years of experience and commitment. Norwegians have been producing and exporting cod liver oil for more than 1000 years. But it was not before 1645 it was reported that cod liver oil could be used to prevent and cure disease. At the end of the 18th century the first scientific article was published to support this. In the middle of the 19th century, the pharmacist Peter Möller observed that people along the west coast of Norway consuming cod liver oil regularly were rarely ill. He dedicated himself to finding out how this healthy liquid could be produced with better taste and pureness at a lower price. He developed a method of using steam to extract the oil from fresh cod livers. Based on this technological advance, the company Peter Möller was founded in 1854 in Lofoten on Norway’s arctic coast, where you find pure, cold, clean seas and high quality raw material. Peter Joachim Möller (1793-1869) At first Möller’s Cod Liver Oil was believed to be a good source of vitamin D and A, and the health benefits were associated with these vitamins. Peter Möller believed, however, that there were other significant benefits from fatty acids and other ingredients in the cod liver oil – both known and unknown. Peter Möller was dedicated to understanding more about these benefits. His dedication and commitment is clear in Möller's vision to improve people’s health by delivering the highest quality omega 3 products. Timeline 1793 Peter Möller is born in Røros, Norway 26 April. 1819 Peter Möller travels to Christiania (Oslo) and is employed by the pharmacist Frantz Peckel at the Svane chemist. He is employed on condition that he passes his pharmaceutical exam within one year. 1822 Graduated as a pharmacist with a unanimous first grade and with the award of the Professor's special satisfaction. 1842 Together with professors A. Holst and Chr. Boeck, Peter Möller participates in the commission which develops the first Norwegian Pharmacopoeia. 1853 Peter announces his method to cod liver oil works along the coast. He equips cod liver oil factories with new equipment in Lofoten, Ålesund and Kristiansund. The facility outside Ålesund is the most important for testing the method. 1854 The Peter Möller company is established as production has started at the three factories. Sales are lower than anticipated even though the quality is considerably better with the new method. The consumers of cod liver oil had been used to the fact that “good medicine must taste bad” and would not believe that the new and better quality was as healthy. Therefore, the following years are used to introduce consumers to the product, and also to convert more producers to the new method. 1869 Peter Möller dies. There are 70 cod liver oil steamers which use his steam rendering method, and 5000 barrels are produced every year. Möller’s company increases the quality by better routines for quality controls. 1870 Severin A Heyerdal, Möller’s son-in-law, assumes the leadership of the firm after Peter's death. He continues the work by improving the quality of the cod liver oil. The goal was to make it as pure and unaltered as in the liver. At this time, Möller had already started selling its product in the USA. In 1870, WH Schieffelin & Co. ("The oldest drughouse in America") was engaged by Peter Möller in the USA. 1881 Frantz Peckel Möller assumes the leadership of the Peter Möller company. He saw it as his duty to further the work on cod liver oil, and through a combination of solid scientific education and an eminent sense of the great mercantile possibilities, he made Möller’s cod liver oil the number one in the world market. 1914 The first world war leads to Möller’s bottled cod liver oil being shut out of the export market. However, domestic sales are good. 1924 The subsidiary Møystad Möller & Co. is established for bulk exports and the Association of Medicinal Cod liver oil Exporters is established in Bergen in 1925. 1925/26 The green bottles are introduced. Medicinal cod liver oil exports remain almost constant, while total Norwegian cod liver oil exports increase. 1938 The factory on the Løren grounds in Oslo, Norway is built. The factory is in the same place today. Peter Möller’s Pharmaceutical Laboratorium A/S is also established to separate out the scientific business. Investment is made in a new facility for refining and bottling veterinary cod liver oil, and increased production of industrial cod liver oil. 1940 The outbreak of the 2nd world war sees exports fall dramatically, while cod liver oil’s significance as a dietary supplement receives increased attention. Domestic sales increase strongly. 1945 After the war, medicinal cod liver oil retains its high status as an important dietary supplement in the “rebuilding" of the country. Cod liver oil becomes an ”emergency product in ravaged areas where the supply situation is difficult. Competition from other countries such as the USA, England and Iceland increases, and Norway no longer dominates the market. 1983 Möller’s cod liver oil in capsule form is launched and palatable cod liver oil is launched. 1990 Peter Möller A/S merges with Orkla Borregaard A/S (now ORKLA) 2005 Peter Möller merges with CollettPharma. The new company is called MöllerCollett. 2007 Merger between MöllerCollet and DanskDroge. The new company is called Axellus. Oval in section with a thin neck, mauve tinted clear glass bottle with text embossed on side.On side : 'P.MOLLER', 'OL JECOR', 'GADOR VER', 'CHRISTIANIA'.cod liver oil, norway, peter moller, christiana, oslo -
 Federation University Historical Collection
Federation University Historical CollectionPhotograph - Photograph - Colour, Installation of University of Ballarat Chancellor Paul Hemming, 2012, 17/0/2012
In April 2012 former Royal Australian College of General Practitioners president Dr Paul Hemming was appointed the University of Ballarat’s new chancellor. Dr Hemming was a deputy chancellor of the university since 2011 and a member of the university council since 2007, and replaces outgoing chancellor Robert Smith. He was a founding director of Beyondblue and has been president of the Royal Australian College of General Practitioners. He was also president of the Australian Medical Association Victoria and has served on numerous federal government medical bodies and tribunals. Chancellors are appointed to chair the university's council (governing body) as the senior office holder of the University. They also confer the academic awards of the university, and represent the university at meetings, functions and ceremonies. At the installation of Dr Hemming as the Fifth Chancellor Of The University Of Ballarat Governor of Victoria Alex Chrnov said: "I join Bonnie Fagan in acknowledging the traditional owners of this land and I pay my respects to their elders past and present. It is my great pleasure, as Governor of Victoria and Visitor to the University of Ballarat, to install Dr Paul Hemming as the fifth Chancellor of this University. I extend to him and his family my warmest congratulations on this appointment and wish him all the best in his new role. I have no doubt that he will be an industrious and wise leader of this University. Although the University of Ballarat is one of Australia’s newest Universities, it is the third oldest Tertiary institution in our country with the School of Mines being established in 1870. It has much to be proud of, and should be recognised for its commitment to being one of our most outstanding regionally focused higher education institutions. It offers on six campuses a diverse, yet suitably targeted, group of learning models that include higher education, TAFE and senior secondary school education, and is one of the few universities in the country that has an associated Technology Park. This University is uniquely placed to provide higher education in Regional Victoria. For example, I understand that by 2020 the population in the Ballarat area will increase by 20% so this University will have the responsibility and opportunity to provide educational opportunities for this growing population. The role of the Governor can be divided into three parts – ceremonial, constitutional and community engagement. It is the third aspect of the role that is most time consuming and, I add, enjoyable. It includes making official visits to Regional areas of Victoria. To date Elizabeth and I have been on 16 such visits and the thing that stands out is that despite the challenges that face Regional communities whether it be through natural disasters, or the economy more broadly – the stoicism, volunteerism and self reliance are always present. Ballarat is an example of such resilience and confidence in its future that can be dated back to this University’s inception. When the School of Mines was established in 1870 on the back of the gold rush era of the 1850s, the local community had vision and faith in its future that is reflected in the building of this institution. It is not dissimilar to Melbourne, where its relatively few citizens established the iconic pillars of our society like the State Library, the University of Melbourne, Parliament Houses and the National Gallery of Victoria. The contribution by the University of Ballarat to Regional Victoria cannot be overstated. Not only does it provide top educational opportunities for students from the Region but its graduates almost invariably end up working in Regional Australia, and often in their own local communities. More specifically, almost three out of every four of the graduates from this University end up finding employment in Regional areas. Such figures highlight the University’s significant contribution to the Regional economy. But its impact is not limited to our Regional areas – it extends to other parts of Australia and overseas. But like so many other higher education institutions in Australia, the University of Ballarat is facing challenges brought about by events such as global uncertainties and the high Australian dollar that impact on the inflow of international students, and dealing with students, more and more of whom come from the lower socio economic sector. It is in those circumstances that the Chancellor must show leadership that involves, amongst others, objectively guiding the Council and supporting the Vice-Chancellor, albeit without becoming involved in the day to day micro management of the University. A strong, trusting and respectful working relationship between the Chancellor and the Vice-Chancellor is, I believe, critical to the sound progress of a University. Before I turn more specifically to Dr Hemming, I would like to reflect briefly on his immediate predecessor, Emeritus Professor Robert Smith. I am sure that Dr Hemming has already found in Professor Smith an invaluable source of assistance. He was a skilled and effective leader not only here, but also in the broader higher education sector. I mention by way of example his instigation and leadership two years ago of the much acclaimed Chancellors’ Conference that was held in Melbourne. There was great diffidence amongst the Chancellors in having it at all. It was a little like herding cats. But Bob Smith spearheaded the organisation of it, with great attention to detail. And it was his hard work and leadership that resulted in the Conference being such a success and of assistance to all Chancellors who attended. It was an illustration of Bob Smith’s skills as a leader in the sector and of this University. And the sector, just as this University remains indebted to him. And I have no doubt that Dr Hemming will similarly lead this University through the challenging, yet exciting, times that lie ahead. He is eminently qualified to do so, in terms of his personal attributes, academic achievements and experience in governance. With his extensive medical career as a General Practitioner, service on a number of Federal Government medical boards and tribunals, and having been a Founding Director of ‘Beyondblue’, President of the Royal Australian College of General Practitioners and President of the Australian Medical Association (Victoria), his list of personal and professional achievements, as well as his strong sense of public and community duty, is impressive. Importantly, Dr Hemming has a long standing connection with the Ballarat community, having moved here with his family from the United Kingdom in 1977. He is now even accepted as a “local” I am told. Given his range of experience to which I have referred and the time he has already spent on the Council and Standing Committees of this University, he is obviously well placed to take part in leading this University. So it is a great pleasure for me to install Dr Hemming as the fifth Chancellor of the University of Ballarat." (http://www.governor.vic.gov.au/victorias-governor/publications/speeches/speech/speech/104) Colour photographS of three men in academic regalia sitting inside the Ballarat Uniting Church, Lydiard Street South. Chancellor Dr Paul Hemming sit in the centre, with Vice-Chancellor Professor david Battersby on the left. Also audience images, academics and a dinner at Craig's Hotel.university of ballarat, federation university, regalia, chancellor, vice chancellor, paul hemming, david battersby, alex chrnov, todd walker, andy smith, craig's hotel, academics -
 Victorian Harness Racing Heritage Collection at Lord's Raceway Bendigo
Victorian Harness Racing Heritage Collection at Lord's Raceway BendigoClothing - Race Colours, Kevin Innes
KEVIN ‘BOOFA’ INNES By Lucy McCormick Kevin was a member of the celebrated Innes clan from Inglewood, in Central Victoria. “I think the first Innes’ came to Inglewood in 1851. My daughter used to say she can’t marry anyone from Inglewood, because she’s related to them all,” says Kevin. ‘Boofa’ is enjoying some well-earned relaxation on the couch after breaking a kneecap six or seven months ago in a track work incident. Not that it seems to be bothering him too much; he’s got plenty of time to keep up with the trots on television. “I do follow them,” Kevin says. “I don’t miss many, and I do have a bet. I like to sit in the chair and drive a race as much as anyone.” With an illustrious career both as a trainer and in the sulky, it’s a safe bet that Kevin Innes is a more than handy ‘grandstand driver’. His name is associated as a trainer/driver with many handy horses, including Lea Sands, Imatoff and Stormy Morn to name a few. Kevin is typically circumspect about his bigger triumphs, however that doesn’t seem to be what interests him the most. “I’ll tell you something,” he declares, doing just that, “I like winning with the horses that were no good. Some people never get a good horse. Imagine that. Luck is a very, very important thing. You have to have luck to buy a good horse at the sales, to get it going, keep it sound, find a race for it, find and owner and get a draw. And they still make a liar of you.” Funny, interesting or quirky stories seem to be of greater interest to Kevin, such as the time he had a strong chance in a standing start race – the favourite in the race being his only worry. “I told the owner it only had a 20-metre handicap – I couldn’t beat it off that,” he remembers. “So I was leading, waiting for the favourite to run past me. Toward the finish, I heard it coming, and it ran straight past all right – minus the driver. He’d fallen out of the cart and I won the race. Just lucky.” The Innes family have always been heavily involved in one sporting pursuit or another – Kevin himself being a champion bike rider of his time. “My Uncle Roy was a good bike rider, so he dared me to have a go. It turned out I was quite good at it as well.” So good, in fact that for many years Kevin was able to make a living from bike riding, riding the ‘board track’ for many years. “We trained hard. Bike riding was very big back then, we’d train and ride three or four times a week.” Kevin’s riding career spanned four Herald Sun Tours, a Warrnambool to Melbourne and a Sydney to Melbourne race, to name a few. “It definitely gets you in – it was long hours,” he muses. “But like anything, horse racing included, you only get back what you put in. We trained hard. I never drank, and I still don’t. I’ve seen that many athletes, great ones too, brought down by alcohol.” Lucky with injury too, Kevin can only remember a sore ankle – as well as the requisite scrapes and abrasions from tumbles on the wooden boards of the velodromes. He still enjoys watching all the big bike races when he can. “You can watch them race all over the world – France, Sweden, Germany. “To be honest I sit up and watch them with my son and we get just as much of a kick looking at the countryside than anything else. It’s so different to when I was racing.” Betting on the bike racing was big in Kevin’s day as well, and some of the bookies Kevin saw betting on the bike racing, he saw at the Showgrounds betting on the trots on a Friday or Saturday night. “Racing was different back then. There would be twelve thousand people at the showgrounds – they don’t have to come anymore, it’s just as easy to watch it on the TV.” Kevin remembers in those days that drivers had to ‘weigh in’ as well – everyone who drove needed to weigh ten stone (just under 65 kilograms). It’s something he remembers fondly. “I know not everyone will.” Kevin won’t be drawn on the subject of favourite drivers, either. “Look. Driving is different now. No disrespect to current drivers, but you had to think a lot more on a three furlong track than they do now on the bigger tracks. You had to drive with brains. And I really believe that good horses make good drivers. The horses are very good these days. Today’s drivers – your Gavin Langs, Chris Alfords – they’re thinkers, and brains will beat brawn every time. The girls are just as good now too. You only have to look at Kerryn Manning.” A garrulous and popular character, Kevin has trained horses for the likes of legendary Richmond player Jack Dyer, and also spent his fair share of time hosting sportsman’s nights, holding his own with the likes of Ron Barassi. On one such night, they had flown in a light aircraft to their destination. During their show, Kevin noticed their pilot, sitting in the front row, laughing appreciatively. There was one problem. He had a beer in his hand, and was consuming it with some enthusiasm. As the night wore on, the pilot became more and more inebriated, and Kevin became more and more disturbed, knowing that this was the same pilot who was to fly them home when they finished. Unbeknownst to Kevin, however, the flight had been cancelled and the pilot hadn’t told anyone, instead deciding to take full advantage of his client’s hospitality. For now, Kevin is happy living in Inglewood with partner Barbara. Son Grant and daughter Carla aren’t far away (both work at the Bendigo Harness track, and Carla has held both a trainer and driver’s licence). His granddaughter, Barclay Sands, was born on the same day of the demise of their star performer, Lea Sands, and may give the biggest hint yet just how important the world of harness racing is to Kevin ‘Boofa’ Innes. Blue with white yolkKevin Innes embroidered on left side chestkevin innes, k innes, bendigo harness racing club, bhrc, bendigo, horses, race colours, trotting, pacing, harness racing -
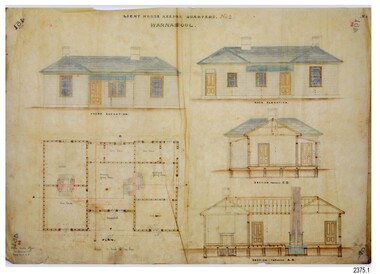 Flagstaff Hill Maritime Museum and Village
Flagstaff Hill Maritime Museum and VillageDocument - Plans, Lighthouse Keeper's Quarters Warrnambool, 1858-1909
The set of seven 1858 plans shows proposed plans for the original Lighthouse Complex that was built on Middle Island in 1858-1859. The whole complex was then transferred to Flagstaff Hill in 1871. The plan, dated 1909, is for proposed additions and repairs to the Quarters at Flagstaff Hill. The plan 'Alterations and Additions' shows alternate plans for changes to the quarters at the Flagstaff Hill location. This plan has no date. The Complex comprised the Lighthouse, the Lighthouse Keepers’ Quarters, the Store (now called the Chart Room) and a Privy, which was not included in these plans. The Keeper’s bluestone Quarters was a cottage divided into two compartments, one for each keeper and his family. The bluestone Store was divided into three; a store, a workshop an oil store (or office). The Privy comprised of a small building also divided into two separate, back-to-back toilets, one for each Keeper and his family. The Flagstaff Hill Keeper's Quarters have had extensions and additions at various times, and these have also been removed at various other times. THE PLANS - *Dec. 1858 (12/58) ‘Lighthouse Keepers Quarters No.2 Warrnambool’ (2375.01)* Public Works Office Melbourne – Front and Back elevations, sections, and floor plan. The drawing shows timber walls. [The floor plan is the closest plan to the current building, however, the walls are timber in this plan.] *Nov. 1858 – No.3 ‘Lighthouse Keepers Quarters Warrnambool’ (2375.02)* Office of Public Works, Melbourne – Back and End elevations and section through. The drawing shows stone walls. One side; Senior Keeper’s bedroom, living room and kitchen with storeroom. Another side; is the Assistant’s bedroom, living room and storeroom. *Nov. 1858 - No.4 ‘Lighthouse Stores Warrnambool’ (2375.03)* Office of Public Works – Front, Side and end elevations, centre section. The drawing shows stone walls. *Nov. 1858 – No.4, ‘Lighthouse Stores No. 2 Warrnambool’ (2375.04)* Office of Public Works – Front, side and end elevations, centre section. The drawing shows timber walls. *Nov. 1858 – ‘Details Lighthouse Keepers Quarters No. 2 Warrnambool’ (2375.05)* Public Works Office Melbourne. The plan shows the foundations, joists and eaves. The drawing shows timber walls. (Nov. 1858 – ‘No.4 ‘Lighthouse No. 2 – Warrnambool’ (2375.06)* Public Works Melbourne (Part of the paper is missing). This plan shows an octagonal tower, internal stairs, a balcony landing, and a weather vane on top. *November 1858 – No. 1, ‘Lighthouse – Warrnambool’ (2375.07)* Office of Public Works Melbourne. This plan shows a round tower, including the stairs, windows on the tower and the weather vane on the top. *4/3/9 [1909] – ‘Additions and Repairs, Lighthouse Quarters, Warrnambool, General Plan’ (2375.8)* Department of Public Works Melbourne’s official stamp is signed by Croft. It shows the floor plans of the Store, Upper Lighthouse and the Quarters. The Store building has three sections; a Store, Work-Shop and Office, with an internal wall between them and separate entries. The Quarters are divided into two dwellings. The Senior Keeper’s side on the left has fireplaces in two of the three bedrooms and there is a pantry and wash house. The Assistant’s side has no fireplaces in the bedrooms and there’s no pantry or washhouse. These plans include proposed changes to the buildings. The Senior Keeper’s Quarters would have a partition on bedroom 2, a bath with plumbing and drainage, a wall moved and a built-in side porch. The Store would also have a built-in porch. The undated plan 'Additions and Alterations' (2375.9) shows alternative arrangements for water tanks, plumbing and such. WARRNAMBOOL'S LADY BAY LIGHTHOUSES- In the 1800s ships sailing from England to Australia began to use Bass Strait as a faster route to Melbourne. Small navigation errors led to many tragic shipwrecks. From 1848 lighthouses were operating along Victoria’s southern coast as a guide for sailors. Coastal towns such as Warrnambool grew and the exchange of trade and passengers were of great benefit. However, the uncertain weather changes, relatively shallow waters and treacherous, hidden rocky reefs were not suitable for a Harbour and in the 1840s and 1850s there were many shipwrecks in the area, with some even stranded in its Lady Bay harbour. A jetty was built in 1850 and a flagstaff to guide seafarers was placed up high on what became known now as Flagstaff Hill. In November 1857 the Victorian Government recommended that Warrnambool Harbour had beacons and two lighthouses to guide vessels into and out of the Harbour safely. The white light of the Middle Island lighthouse was to be used for the first time on September 1, 1859. The red light of the Beach Lighthouse, a wooden obelisk structure, was first operated on March 25, 1860, but in 1868 this light was ‘discontinued’ due to it being too low. Melbourne’s Department of Public Works decided to relocate the Middle Island Lighthouse Complex - Lighthouse, Keeper’s Quarters, Privy, Store Room and even water tanks - to Flagstaff Hill. The lower obelisk was shortened, and a protruding gallery, railing, and external ladder were added, as well as the light from the Beach Lighthouse. A green guiding light was erected on the end of the jetty. The transfer of the Complex began in March 1871. Each shaped stone of the lighthouse was carefully numbered, removed then reassembled on Flagstaff Hill. In 1872 the well was sunk behind the Lighthouse Keeper’s Cottage. The Keepers and families had left Middle Island in April and moved to Flagstaff Hill in October 1871. Vessels entering Lady Bay align the Upper and Lower Lighthouse towers during the day and the lights at night. The Upper Lighthouse is a round tower, the Lower Light is square. The Lighthouses were categorised as harbour lights rather than coastal lights, so they remain under the control of the Victorian Government’s Ports and Harbours section. The lights were originally powered by oil, then acetylene gas, later by electricity, and then converted to solar power in 1988. In 1993 the solar panel was replaced by a battery charger. A decision was made in 1936 to replace the lighthouses’ lights with unattended lights that no longer required Keepers and Assistants. At least 29 Keepers had attended to the lighthouse from its opening in 1859 to when the last official Lightkeepers left In April 1916. The Warrnambool Harbour Board rented out the Quarters from 1916 to 1936. The Board closed down but the rentals continued with other unknown landlords. In the 1970s the Flagstaff Hill Planning Board was set up under the chairmanship of John Lindsay. The Board was to make recommendations to the Warrnambool City Council regarding the use of the buildings and the rest of the Crown Land on the site. The Flagstaff Hill Maritime Village opened in 1975 and began renovating the Cottage in stages, during which time evidence of a 1920s fire was found in the eastern section of the cottage. Additions of a porch on the west and a washroom on the east were made in the 1980s. The western part of the building is now a Shipwreck Museum and the east has returned to a late 19th-century Lighthouse Keeper’s cottage and includes the screen made by Assistant Lighthouse Keeper Thomas Hope during one of his two periods of service there. THE LIGHTHOUSE KEEPERS Lighthouse Keepers were responsible for keeping their Lighthouse’s lights shining at night. They kept a lookout for passing vessels and changes in weather. They were expected to clean, polish and maintain the equipment and buildings. They kept regular and detailed records of who was on watch, and the time the light was lit, trimmed and extinguished. They kept a journal about other events that occurred. They keep regular, accurate Meteorological Logs. It was expected that they were competent in Morse code signalling. They would be called to help in times of disasters and shipwrecks, and to give official statements about these events. Many Lighthouse Keepers also volunteered as members of the lifeboat crew. The Lady Bay lighthouses were officially classified as small, so the Keepers had the official titles of Senior Assistant Lighthouse Keeper and Assistant Lighthouse Keeper. They were employed by the Public Service and paid rent to live in the Lighthouse Quarters. They were compulsorily retired at the age of 60, with most receiving a superannuation payment. Despite their time-consuming duties, there was time to follow hobbies and crafts such as growing vegetables, playing musical instruments, making models of buildings including lighthouses, and crafting furniture pieces. An example of a keeper’s skills is the carved fire screen made by /assistant Keeper Thomas Hope in the early 20th century and displayed in the Lighthouse Keeper’s cottage at Flagstaff Hill. The last occupants of the Middle Island Complex were Senior Keeper Robert Deverell, his Assistant Keeper, Andrew Farncombe, and their families. They all became the first occupants at the Lady Bay Lighthouse Keepers’ Quarters on Merri Street. The Warrnambool Lighthouse Complex plans are the origin of what is now the Lady Bay Lighthouse Complex. They are a record of the people, process and departments involved in bringing the complex into fruition. The plans are significant to the Complex, which is now listed on the Victorian Heritage Register, H1520, for being of historical, scientific (technological) and architectural significance to the State of Victoria. The Complex is significant as an example of early colonial development. The plan are significant for their connection with the important navigational function of the Lighthouses, a function still being performed to this day. The plans are also significant as an example of a product from the Public Works Department in Victoria in the mid-to-late 19th century. The structures built to these plans still stand strong. Plans for the Lighthouse Complex in Warrnambool, including Lighthouses, Keeper's Quarters and Stores. Seven of the plans are on thin fragile paper, one is on thicker, stronger paper. The drawings have been made in pens coloured red and black. They originate from Public Works in Melbourne. Seven were drawn in 1858, one in 1904, the other is not dated.Dec. 1858 - Lighthouse Keepers Quarters No.2 Warrnambool. Public Works Office Melbourne. Nov. 1858 - No.3 ‘Lighthouse Keepers Quarters Warrnambool. Public Works Office Melbourne. Nov. 1858 - No.4 ‘Lighthouse Stores Warrnambool. Office of Public Works. Nov. 1858 - No.4, ‘Lighthouse Stores No. 2 Warrnambool. Office of Public Works. Nov. 1858 - Details Lighthouse Keepers Quarters No. 2 Warrnambool. Public Works Office Melbourne. Nov. 1858 - No.4 ‘Lighthouse No. 2 – Warrnambool. Public Works Melbourne. Nov. 1858 - No. 1, Lighthouse - Warrnambool. Office of Public Works Melbourne. 4/3/9 [1909] - Additions and Repairs, Lighthouse Quarters, Warrnambool, General Plan. Department of Public Works Melbourne. SIGNED "Croft" "15A" on reverse [no date] - Lighthouse Quarters Warrnambool, Additions and Alterations. "9A" on reverseflagstaff hill, warrnambool, lighthouse keeper's cottage, lighthouse residence, lighthouse, plans, public works, melbourne, flagstaff hill maritime museum, maritime museum, shipwreck coast, flagstaff hill maritime village, chart room, quarters, privy, middle island, beach lighthouse, obelisk, lighthouse keeper, assistant keeper, lighthouse complex, lady bay, lady bay complex, keepers, upper lighthouse, lower lighthouse, ports and harbours, cottage, harbour board, flagstaff hill planning board, meteorological record, robert deverell, andrew farncombe, warrnambool port, warrnambool harbour, residence, alterations, repairs, department of works -
 Flagstaff Hill Maritime Museum and Village
Flagstaff Hill Maritime Museum and VillageAnimal specimen - Whale bone, Undetermined
Prior to carrying out a detailed condition report of the cetacean skeletons, it is useful to have an understanding of the materials we are likely to encounter, in terms of structure and chemistry. This entry invites you to join in learning about the composition of whale bone and oil. Whale bone (Cetacean) bone is comprised of a composite structure of both an inorganic matrix of mainly hydroxylapatite (a calcium phosphate mineral), providing strength and rigidity, as well as an organic protein ‘scaffolding’ of mainly collagen, facilitating growth and repair (O’Connor 2008, CCI 2010). Collagen is also the structural protein component in cartilage between the whale vertebrae and attached to the fins of both the Killer Whale and the Dolphin. Relative proportions in the bone composition (affecting density), are linked with the feeding habits and mechanical stresses typically endured by bones of particular whale types. A Sperm Whale (Physeter macrocephalus Linnaeus, 1758) skeleton (toothed) thus has a higher mineral value (~67%) than a Fin Whale (Balaenoptera physalus Linnaeus, 1758) (baleen) (~60%) (Turner Walker 2012). The internal structure of bone can be divided into compact and cancellous bone. In whales, load-bearing structures such as mandibles and upper limb bones (e.g. humerus, sternum) are largely composed of compact bone (Turner Walker 2012). This consists of lamella concentrically deposited around the longitudinal axis and is permeated by fluid carrying channels (O’Connor 2008). Cancellous (spongy) bone, with a highly porous angular network of trabeculae, is less stiff and thus found in whale ribs and vertebrae (Turner Walker 2012). Whale oil Whales not only carry a thick layer of fat (blubber) in the soft tissue of their body for heat insulation and as a food store while they are alive, but also hold large oil (lipid) reserves in their porous bones. Following maceration of the whale skeleton after death to remove the soft tissue, the bones retain a high lipid content (Higgs et. al 2010). Particularly bones with a spongy (porous) structure have a high capacity to hold oil-rich marrow. Comparative data of various whale species suggests the skull, particularly the cranium and mandible bones are particularly oil rich. Along the vertebral column, the lipid content is reduced, particularly in the thoracic vertebrae (~10-25%), yet greatly increases from the lumbar to the caudal vertebrae (~40-55%). The chest area (scapula, sternum and ribs) show a mid-range lipid content (~15-30%), with vertically orientated ribs being more heavily soaked lower down (Turner Walker 2012, Higgs et. al 2010). Whale oil is largely composed of triglycerides (molecules of fatty acids attached to a glycerol molecule). In Arctic whales a higher proportion of unsaturated, versus saturated fatty acids make up the lipid. Unsaturated fatty acids (with double or triple carbon bonds causing chain kinks, preventing close packing (solidifying) of molecules), are more likely to be liquid (oil), versus solid (fat) at room temperature (Smith and March 2007). Objects Made From the Whaling Industry We all know that men set forth in sailing ships and risked their lives to harpoon whales on the open seas throughout the 1800s. And while Moby Dick and other tales have made whaling stories immortal, people today generally don't appreciate that the whalers were part of a well-organized industry. The ships that set out from ports in New England roamed as far as the Pacific in hunt of specific species of whales. Adventure may have been the draw for some whalers, but for the captains who owned whaling ships, and the investors which financed voyages, there was a considerable monetary payoff. The gigantic carcasses of whales were chopped and boiled down and turned into products such as the fine oil needed to lubricate increasing advanced machine tools. And beyond the oil derived from whales, even their bones, in an era before the invention of plastic, was used to make a wide variety of consumer goods. In short, whales were a valuable natural resource the same as wood, minerals, or petroleum we now pump from the ground. Oil From Whale’s Blubber Oil was the main product sought from whales, and it was used to lubricate machinery and to provide illumination by burning it in lamps. When a whale was killed, it was towed to the ship and its blubber, the thick insulating fat under its skin, would be peeled and cut from its carcass in a process known as “flensing.” The blubber was minced into chunks and boiled in large vats on board the whaling ship, producing oil. The oil taken from whale blubber was packaged in casks and transported back to the whaling ship’s home port (such as New Bedford, Massachusetts, the busiest American whaling port in the mid-1800s). From the ports it would be sold and transported across the country and would find its way into a huge variety of products. Whale oil, in addition to be used for lubrication and illumination, was also used to manufacture soaps, paint, and varnish. Whale oil was also utilized in some processes used to manufacture textiles and rope. Spermaceti, a Highly Regarded Oil A peculiar oil found in the head of the sperm whale, spermaceti, was highly prized. The oil was waxy, and was commonly used in making candles. In fact, candles made of spermaceti were considered the best in the world, producing a bright clear flame without an excess of smoke. Spermaceti was also used, distilled in liquid form, as an oil to fuel lamps. The main American whaling port, New Bedford, Massachusetts, was thus known as "The City That Lit the World." When John Adams was the ambassador to Great Britain before serving as president he recorded in his diary a conversation about spermaceti he had with the British Prime Minister William Pitt. Adams, keen to promote the New England whaling industry, was trying to convince the British to import spermaceti sold by American whalers, which the British could use to fuel street lamps. The British were not interested. In his diary, Adams wrote that he told Pitt, “the fat of the spermaceti whale gives the clearest and most beautiful flame of any substance that is known in nature, and we are surprised you prefer darkness, and consequent robberies, burglaries, and murders in your streets to receiving as a remittance our spermaceti oil.” Despite the failed sales pitch John Adams made in the late 1700s, the American whaling industry boomed in the early to mid-1800s. And spermaceti was a major component of that success. Spermaceti could be refined into a lubricant that was ideal for precision machinery. The machine tools that made the growth of industry possible in the United States were lubricated, and essentially made possible, by oil derived from spermaceti. Baleen, or "Whalebone" The bones and teeth of various species of whales were used in a number of products, many of them common implements in a 19th century household. Whales are said to have produced “the plastic of the 1800s.” The "bone" of the whale which was most commonly used wasn’t technically a bone, it was baleen, a hard material arrayed in large plates, like gigantic combs, in the mouths of some species of whales. The purpose of the baleen is to act as a sieve, catching tiny organisms in sea water, which the whale consumes as food. As baleen was tough yet flexible, it could be used in a number of practical applications. And it became commonly known as "whalebone." Perhaps the most common use of whalebone was in the manufacture of corsets, which fashionable ladies in the 1800s wore to compress their waistlines. One typical corset advertisement from the 1800s proudly proclaims, “Real Whalebone Only Used.” Whalebone was also used for collar stays, buggy whips, and toys. Its remarkable flexibility even caused it to be used as the springs in early typewriters. The comparison to plastic is apt. Think of common items which today might be made of plastic, and it's likely that similar items in the 1800s would have been made of whalebone. Baleen whales do not have teeth. But the teeth of other whales, such as the sperm whale, would be used as ivory in such products as chess pieces, piano keys, or the handles of walking sticks. Pieces of scrimshaw, or carved whale's teeth, would probably be the best remembered use of whale's teeth. However, the carved teeth were created to pass the time on whaling voyages and were never a mass production item. Their relative rarity, of course, is why genuine pieces of 19th century scrimshaw are considered to be valuable collectibles today. Reference: McNamara, Robert. "Objects Made From the Whaling Industry." ThoughtCo, Jul. 31, 2021, thoughtco.com/products-produced-from-whales-1774070.Whale bone was an important commodity, used in corsets, collar stays, buggy whips, and toys.Whale bone in two pieces. Advanced stage of calcification as indicated by deep pitting. Off white to grey.None.flagstaff hill, warrnambool, shipwrecked-coast, flagstaff-hill, flagstaff-hill-maritime-museum, maritime-museum, shipwreck-coast, flagstaff-hill-maritime-village, whale bones, whale skeleton, whales, whale bone, corsets, toys, whips -
 Flagstaff Hill Maritime Museum and Village
Flagstaff Hill Maritime Museum and VillageAnimal specimen - Whale bone, Undetermined
Prior to carrying out a detailed condition report of the cetacean skeletons, it is useful to have an understanding of the materials we are likely to encounter, in terms of structure and chemistry. This entry invites you to join in learning about the composition of whale bone and oil. Whale bone (Cetacean) bone is comprised of a composite structure of both an inorganic matrix of mainly hydroxylapatite (a calcium phosphate mineral), providing strength and rigidity, as well as an organic protein ‘scaffolding’ of mainly collagen, facilitating growth and repair (O’Connor 2008, CCI 2010). Collagen is also the structural protein component in cartilage between the whale vertebrae and attached to the fins of both the Killer Whale and the Dolphin. Relative proportions in the bone composition (affecting density), are linked with the feeding habits and mechanical stresses typically endured by bones of particular whale types. A Sperm Whale (Physeter macrocephalus Linnaeus, 1758) skeleton (toothed) thus has a higher mineral value (~67%) than a Fin Whale (Balaenoptera physalus Linnaeus, 1758) (baleen) (~60%) (Turner Walker 2012). The internal structure of bone can be divided into compact and cancellous bone. In whales, load-bearing structures such as mandibles and upper limb bones (e.g. humerus, sternum) are largely composed of compact bone (Turner Walker 2012). This consists of lamella concentrically deposited around the longitudinal axis and is permeated by fluid carrying channels (O’Connor 2008). Cancellous (spongy) bone, with a highly porous angular network of trabeculae, is less stiff and thus found in whale ribs and vertebrae (Turner Walker 2012). Whale oil Whales not only carry a thick layer of fat (blubber) in the soft tissue of their body for heat insulation and as a food store while they are alive, but also hold large oil (lipid) reserves in their porous bones. Following maceration of the whale skeleton after death to remove the soft tissue, the bones retain a high lipid content (Higgs et. al 2010). Particularly bones with a spongy (porous) structure have a high capacity to hold oil-rich marrow. Comparative data of various whale species suggests the skull, particularly the cranium and mandible bones are particularly oil rich. Along the vertebral column, the lipid content is reduced, particularly in the thoracic vertebrae (~10-25%), yet greatly increases from the lumbar to the caudal vertebrae (~40-55%). The chest area (scapula, sternum and ribs) show a mid-range lipid content (~15-30%), with vertically orientated ribs being more heavily soaked lower down (Turner Walker 2012, Higgs et. al 2010). Whale oil is largely composed of triglycerides (molecules of fatty acids attached to a glycerol molecule). In Arctic whales a higher proportion of unsaturated, versus saturated fatty acids make up the lipid. Unsaturated fatty acids (with double or triple carbon bonds causing chain kinks, preventing close packing (solidifying) of molecules), are more likely to be liquid (oil), versus solid (fat) at room temperature (Smith and March 2007). Objects Made From the Whaling Industry We all know that men set forth in sailing ships and risked their lives to harpoon whales on the open seas throughout the 1800s. And while Moby Dick and other tales have made whaling stories immortal, people today generally don't appreciate that the whalers were part of a well-organized industry. The ships that set out from ports in New England roamed as far as the Pacific in hunt of specific species of whales. Adventure may have been the draw for some whalers, but for the captains who owned whaling ships, and the investors which financed voyages, there was a considerable monetary payoff. The gigantic carcasses of whales were chopped and boiled down and turned into products such as the fine oil needed to lubricate increasing advanced machine tools. And beyond the oil derived from whales, even their bones, in an era before the invention of plastic, was used to make a wide variety of consumer goods. In short, whales were a valuable natural resource the same as wood, minerals, or petroleum we now pump from the ground. Oil From Whale’s Blubber Oil was the main product sought from whales, and it was used to lubricate machinery and to provide illumination by burning it in lamps. When a whale was killed, it was towed to the ship and its blubber, the thick insulating fat under its skin, would be peeled and cut from its carcass in a process known as “flensing.” The blubber was minced into chunks and boiled in large vats on board the whaling ship, producing oil. The oil taken from whale blubber was packaged in casks and transported back to the whaling ship’s home port (such as New Bedford, Massachusetts, the busiest American whaling port in the mid-1800s). From the ports it would be sold and transported across the country and would find its way into a huge variety of products. Whale oil, in addition to be used for lubrication and illumination, was also used to manufacture soaps, paint, and varnish. Whale oil was also utilized in some processes used to manufacture textiles and rope. Spermaceti, a Highly Regarded Oil A peculiar oil found in the head of the sperm whale, spermaceti, was highly prized. The oil was waxy, and was commonly used in making candles. In fact, candles made of spermaceti were considered the best in the world, producing a bright clear flame without an excess of smoke. Spermaceti was also used, distilled in liquid form, as an oil to fuel lamps. The main American whaling port, New Bedford, Massachusetts, was thus known as "The City That Lit the World." When John Adams was the ambassador to Great Britain before serving as president he recorded in his diary a conversation about spermaceti he had with the British Prime Minister William Pitt. Adams, keen to promote the New England whaling industry, was trying to convince the British to import spermaceti sold by American whalers, which the British could use to fuel street lamps. The British were not interested. In his diary, Adams wrote that he told Pitt, “the fat of the spermaceti whale gives the clearest and most beautiful flame of any substance that is known in nature, and we are surprised you prefer darkness, and consequent robberies, burglaries, and murders in your streets to receiving as a remittance our spermaceti oil.” Despite the failed sales pitch John Adams made in the late 1700s, the American whaling industry boomed in the early to mid-1800s. And spermaceti was a major component of that success. Spermaceti could be refined into a lubricant that was ideal for precision machinery. The machine tools that made the growth of industry possible in the United States were lubricated, and essentially made possible, by oil derived from spermaceti. Baleen, or "Whalebone" The bones and teeth of various species of whales were used in a number of products, many of them common implements in a 19th century household. Whales are said to have produced “the plastic of the 1800s.” The "bone" of the whale which was most commonly used wasn’t technically a bone, it was baleen, a hard material arrayed in large plates, like gigantic combs, in the mouths of some species of whales. The purpose of the baleen is to act as a sieve, catching tiny organisms in sea water, which the whale consumes as food. As baleen was tough yet flexible, it could be used in a number of practical applications. And it became commonly known as "whalebone." Perhaps the most common use of whalebone was in the manufacture of corsets, which fashionable ladies in the 1800s wore to compress their waistlines. One typical corset advertisement from the 1800s proudly proclaims, “Real Whalebone Only Used.” Whalebone was also used for collar stays, buggy whips, and toys. Its remarkable flexibility even caused it to be used as the springs in early typewriters. The comparison to plastic is apt. Think of common items which today might be made of plastic, and it's likely that similar items in the 1800s would have been made of whalebone. Baleen whales do not have teeth. But the teeth of other whales, such as the sperm whale, would be used as ivory in such products as chess pieces, piano keys, or the handles of walking sticks. Pieces of scrimshaw, or carved whale's teeth, would probably be the best remembered use of whale's teeth. However, the carved teeth were created to pass the time on whaling voyages and were never a mass production item. Their relative rarity, of course, is why genuine pieces of 19th century scrimshaw are considered to be valuable collectibles today. Reference: McNamara, Robert. "Objects Made From the Whaling Industry." ThoughtCo, Jul. 31, 2021, thoughtco.com/products-produced-from-whales-1774070.Whale bone was an important commodity, used in corsets, collar stays, buggy whips, and toys.Whale bone piece. Advanced stage of calcification as indicated by deep pitting. Off white to grey.None.flagstaff hill, warrnambool, shipwrecked-coast, flagstaff-hill, flagstaff-hill-maritime-museum, maritime-museum, shipwreck-coast, flagstaff-hill-maritime-village, whales, whale bone, corsets, toys, whips -
 Flagstaff Hill Maritime Museum and Village
Flagstaff Hill Maritime Museum and VillageAnimal specimen - Whale bone, Undetermined
Prior to carrying out a detailed condition report of the cetacean skeletons, it is useful to have an understanding of the materials we are likely to encounter, in terms of structure and chemistry. This entry invites you to join in learning about the composition of whale bone and oil. Whale bone (Cetacean) bone is comprised of a composite structure of both an inorganic matrix of mainly hydroxylapatite (a calcium phosphate mineral), providing strength and rigidity, as well as an organic protein ‘scaffolding’ of mainly collagen, facilitating growth and repair (O’Connor 2008, CCI 2010). Collagen is also the structural protein component in cartilage between the whale vertebrae and attached to the fins of both the Killer Whale and the Dolphin. Relative proportions in the bone composition (affecting density), are linked with the feeding habits and mechanical stresses typically endured by bones of particular whale types. A Sperm Whale (Physeter macrocephalus Linnaeus, 1758) skeleton (toothed) thus has a higher mineral value (~67%) than a Fin Whale (Balaenoptera physalus Linnaeus, 1758) (baleen) (~60%) (Turner Walker 2012). The internal structure of bone can be divided into compact and cancellous bone. In whales, load-bearing structures such as mandibles and upper limb bones (e.g. humerus, sternum) are largely composed of compact bone (Turner Walker 2012). This consists of lamella concentrically deposited around the longitudinal axis and is permeated by fluid carrying channels (O’Connor 2008). Cancellous (spongy) bone, with a highly porous angular network of trabeculae, is less stiff and thus found in whale ribs and vertebrae (Turner Walker 2012). Whale oil Whales not only carry a thick layer of fat (blubber) in the soft tissue of their body for heat insulation and as a food store while they are alive, but also hold large oil (lipid) reserves in their porous bones. Following maceration of the whale skeleton after death to remove the soft tissue, the bones retain a high lipid content (Higgs et. al 2010). Particularly bones with a spongy (porous) structure have a high capacity to hold oil-rich marrow. Comparative data of various whale species suggests the skull, particularly the cranium and mandible bones are particularly oil rich. Along the vertebral column, the lipid content is reduced, particularly in the thoracic vertebrae (~10-25%), yet greatly increases from the lumbar to the caudal vertebrae (~40-55%). The chest area (scapula, sternum and ribs) show a mid-range lipid content (~15-30%), with vertically orientated ribs being more heavily soaked lower down (Turner Walker 2012, Higgs et. al 2010). Whale oil is largely composed of triglycerides (molecules of fatty acids attached to a glycerol molecule). In Arctic whales a higher proportion of unsaturated, versus saturated fatty acids make up the lipid. Unsaturated fatty acids (with double or triple carbon bonds causing chain kinks, preventing close packing (solidifying) of molecules), are more likely to be liquid (oil), versus solid (fat) at room temperature (Smith and March 2007). Objects Made From the Whaling Industry We all know that men set forth in sailing ships and risked their lives to harpoon whales on the open seas throughout the 1800s. And while Moby Dick and other tales have made whaling stories immortal, people today generally don't appreciate that the whalers were part of a well-organized industry. The ships that set out from ports in New England roamed as far as the Pacific in hunt of specific species of whales. Adventure may have been the draw for some whalers, but for the captains who owned whaling ships, and the investors which financed voyages, there was a considerable monetary payoff. The gigantic carcasses of whales were chopped and boiled down and turned into products such as the fine oil needed to lubricate increasing advanced machine tools. And beyond the oil derived from whales, even their bones, in an era before the invention of plastic, was used to make a wide variety of consumer goods. In short, whales were a valuable natural resource the same as wood, minerals, or petroleum we now pump from the ground. Oil From Whale’s Blubber Oil was the main product sought from whales, and it was used to lubricate machinery and to provide illumination by burning it in lamps. When a whale was killed, it was towed to the ship and its blubber, the thick insulating fat under its skin, would be peeled and cut from its carcass in a process known as “flensing.” The blubber was minced into chunks and boiled in large vats on board the whaling ship, producing oil. The oil taken from whale blubber was packaged in casks and transported back to the whaling ship’s home port (such as New Bedford, Massachusetts, the busiest American whaling port in the mid-1800s). From the ports it would be sold and transported across the country and would find its way into a huge variety of products. Whale oil, in addition to be used for lubrication and illumination, was also used to manufacture soaps, paint, and varnish. Whale oil was also utilized in some processes used to manufacture textiles and rope. Spermaceti, a Highly Regarded Oil A peculiar oil found in the head of the sperm whale, spermaceti, was highly prized. The oil was waxy, and was commonly used in making candles. In fact, candles made of spermaceti were considered the best in the world, producing a bright clear flame without an excess of smoke. Spermaceti was also used, distilled in liquid form, as an oil to fuel lamps. The main American whaling port, New Bedford, Massachusetts, was thus known as "The City That Lit the World." When John Adams was the ambassador to Great Britain before serving as president he recorded in his diary a conversation about spermaceti he had with the British Prime Minister William Pitt. Adams, keen to promote the New England whaling industry, was trying to convince the British to import spermaceti sold by American whalers, which the British could use to fuel street lamps. The British were not interested. In his diary, Adams wrote that he told Pitt, “the fat of the spermaceti whale gives the clearest and most beautiful flame of any substance that is known in nature, and we are surprised you prefer darkness, and consequent robberies, burglaries, and murders in your streets to receiving as a remittance our spermaceti oil.” Despite the failed sales pitch John Adams made in the late 1700s, the American whaling industry boomed in the early to mid-1800s. And spermaceti was a major component of that success. Spermaceti could be refined into a lubricant that was ideal for precision machinery. The machine tools that made the growth of industry possible in the United States were lubricated, and essentially made possible, by oil derived from spermaceti. Baleen, or "Whalebone" The bones and teeth of various species of whales were used in a number of products, many of them common implements in a 19th century household. Whales are said to have produced “the plastic of the 1800s.” The "bone" of the whale which was most commonly used wasn’t technically a bone, it was baleen, a hard material arrayed in large plates, like gigantic combs, in the mouths of some species of whales. The purpose of the baleen is to act as a sieve, catching tiny organisms in sea water, which the whale consumes as food. As baleen was tough yet flexible, it could be used in a number of practical applications. And it became commonly known as "whalebone." Perhaps the most common use of whalebone was in the manufacture of corsets, which fashionable ladies in the 1800s wore to compress their waistlines. One typical corset advertisement from the 1800s proudly proclaims, “Real Whalebone Only Used.” Whalebone was also used for collar stays, buggy whips, and toys. Its remarkable flexibility even caused it to be used as the springs in early typewriters. The comparison to plastic is apt. Think of common items which today might be made of plastic, and it's likely that similar items in the 1800s would have been made of whalebone. Baleen whales do not have teeth. But the teeth of other whales, such as the sperm whale, would be used as ivory in such products as chess pieces, piano keys, or the handles of walking sticks. Pieces of scrimshaw, or carved whale's teeth, would probably be the best remembered use of whale's teeth. However, the carved teeth were created to pass the time on whaling voyages and were never a mass production item. Their relative rarity, of course, is why genuine pieces of 19th century scrimshaw are considered to be valuable collectibles today. Reference: McNamara, Robert. "Objects Made From the Whaling Industry." ThoughtCo, Jul. 31, 2021, thoughtco.com/products-produced-from-whales-1774070. Whale bone was an important commodity, used in corsets, collar stays, buggy whips, and toys.Whale bone vertebrae. Advanced stage of calcification as indicated by deep pitting. Off white to grey.None.flagstaff hill, warrnambool, shipwrecked-coast, flagstaff-hill, flagstaff-hill-maritime-museum, maritime-museum, shipwreck-coast, flagstaff-hill-maritime-village, whales, whale bone, corsets, toys, whips -
 Flagstaff Hill Maritime Museum and Village
Flagstaff Hill Maritime Museum and VillageAnimal specimen - Whale bone, Undetermined
Prior to carrying out a detailed condition report of the cetacean skeletons, it is useful to have an understanding of the materials we are likely to encounter, in terms of structure and chemistry. This entry invites you to join in learning about the composition of whale bone and oil. Whale bone (Cetacean) bone is comprised of a composite structure of both an inorganic matrix of mainly hydroxylapatite (a calcium phosphate mineral), providing strength and rigidity, as well as an organic protein ‘scaffolding’ of mainly collagen, facilitating growth and repair (O’Connor 2008, CCI 2010). Collagen is also the structural protein component in cartilage between the whale vertebrae and attached to the fins of both the Killer Whale and the Dolphin. Relative proportions in the bone composition (affecting density), are linked with the feeding habits and mechanical stresses typically endured by bones of particular whale types. A Sperm Whale (Physeter macrocephalus Linnaeus, 1758) skeleton (toothed) thus has a higher mineral value (~67%) than a Fin Whale (Balaenoptera physalus Linnaeus, 1758) (baleen) (~60%) (Turner Walker 2012). The internal structure of bone can be divided into compact and cancellous bone. In whales, load-bearing structures such as mandibles and upper limb bones (e.g. humerus, sternum) are largely composed of compact bone (Turner Walker 2012). This consists of lamella concentrically deposited around the longitudinal axis and is permeated by fluid carrying channels (O’Connor 2008). Cancellous (spongy) bone, with a highly porous angular network of trabeculae, is less stiff and thus found in whale ribs and vertebrae (Turner Walker 2012). Whale oil Whales not only carry a thick layer of fat (blubber) in the soft tissue of their body for heat insulation and as a food store while they are alive, but also hold large oil (lipid) reserves in their porous bones. Following maceration of the whale skeleton after death to remove the soft tissue, the bones retain a high lipid content (Higgs et. al 2010). Particularly bones with a spongy (porous) structure have a high capacity to hold oil-rich marrow. Comparative data of various whale species suggests the skull, particularly the cranium and mandible bones are particularly oil rich. Along the vertebral column, the lipid content is reduced, particularly in the thoracic vertebrae (~10-25%), yet greatly increases from the lumbar to the caudal vertebrae (~40-55%). The chest area (scapula, sternum and ribs) show a mid-range lipid content (~15-30%), with vertically orientated ribs being more heavily soaked lower down (Turner Walker 2012, Higgs et. al 2010). Whale oil is largely composed of triglycerides (molecules of fatty acids attached to a glycerol molecule). In Arctic whales a higher proportion of unsaturated, versus saturated fatty acids make up the lipid. Unsaturated fatty acids (with double or triple carbon bonds causing chain kinks, preventing close packing (solidifying) of molecules), are more likely to be liquid (oil), versus solid (fat) at room temperature (Smith and March 2007). Objects Made From the Whaling Industry We all know that men set forth in sailing ships and risked their lives to harpoon whales on the open seas throughout the 1800s. And while Moby Dick and other tales have made whaling stories immortal, people today generally don't appreciate that the whalers were part of a well-organized industry. The ships that set out from ports in New England roamed as far as the Pacific in hunt of specific species of whales. Adventure may have been the draw for some whalers, but for the captains who owned whaling ships, and the investors which financed voyages, there was a considerable monetary payoff. The gigantic carcasses of whales were chopped and boiled down and turned into products such as the fine oil needed to lubricate increasing advanced machine tools. And beyond the oil derived from whales, even their bones, in an era before the invention of plastic, was used to make a wide variety of consumer goods. In short, whales were a valuable natural resource the same as wood, minerals, or petroleum we now pump from the ground. Oil From Whale’s Blubber Oil was the main product sought from whales, and it was used to lubricate machinery and to provide illumination by burning it in lamps. When a whale was killed, it was towed to the ship and its blubber, the thick insulating fat under its skin, would be peeled and cut from its carcass in a process known as “flensing.” The blubber was minced into chunks and boiled in large vats on board the whaling ship, producing oil. The oil taken from whale blubber was packaged in casks and transported back to the whaling ship’s home port (such as New Bedford, Massachusetts, the busiest American whaling port in the mid-1800s). From the ports it would be sold and transported across the country and would find its way into a huge variety of products. Whale oil, in addition to be used for lubrication and illumination, was also used to manufacture soaps, paint, and varnish. Whale oil was also utilized in some processes used to manufacture textiles and rope. Spermaceti, a Highly Regarded Oil A peculiar oil found in the head of the sperm whale, spermaceti, was highly prized. The oil was waxy, and was commonly used in making candles. In fact, candles made of spermaceti were considered the best in the world, producing a bright clear flame without an excess of smoke. Spermaceti was also used, distilled in liquid form, as an oil to fuel lamps. The main American whaling port, New Bedford, Massachusetts, was thus known as "The City That Lit the World." When John Adams was the ambassador to Great Britain before serving as president he recorded in his diary a conversation about spermaceti he had with the British Prime Minister William Pitt. Adams, keen to promote the New England whaling industry, was trying to convince the British to import spermaceti sold by American whalers, which the British could use to fuel street lamps. The British were not interested. In his diary, Adams wrote that he told Pitt, “the fat of the spermaceti whale gives the clearest and most beautiful flame of any substance that is known in nature, and we are surprised you prefer darkness, and consequent robberies, burglaries, and murders in your streets to receiving as a remittance our spermaceti oil.” Despite the failed sales pitch John Adams made in the late 1700s, the American whaling industry boomed in the early to mid-1800s. And spermaceti was a major component of that success. Spermaceti could be refined into a lubricant that was ideal for precision machinery. The machine tools that made the growth of industry possible in the United States were lubricated, and essentially made possible, by oil derived from spermaceti. Baleen, or "Whalebone" The bones and teeth of various species of whales were used in a number of products, many of them common implements in a 19th century household. Whales are said to have produced “the plastic of the 1800s.” The "bone" of the whale which was most commonly used wasn’t technically a bone, it was baleen, a hard material arrayed in large plates, like gigantic combs, in the mouths of some species of whales. The purpose of the baleen is to act as a sieve, catching tiny organisms in sea water, which the whale consumes as food. As baleen was tough yet flexible, it could be used in a number of practical applications. And it became commonly known as "whalebone." Perhaps the most common use of whalebone was in the manufacture of corsets, which fashionable ladies in the 1800s wore to compress their waistlines. One typical corset advertisement from the 1800s proudly proclaims, “Real Whalebone Only Used.” Whalebone was also used for collar stays, buggy whips, and toys. Its remarkable flexibility even caused it to be used as the springs in early typewriters. The comparison to plastic is apt. Think of common items which today might be made of plastic, and it's likely that similar items in the 1800s would have been made of whalebone. Baleen whales do not have teeth. But the teeth of other whales, such as the sperm whale, would be used as ivory in such products as chess pieces, piano keys, or the handles of walking sticks. Pieces of scrimshaw, or carved whale's teeth, would probably be the best remembered use of whale's teeth. However, the carved teeth were created to pass the time on whaling voyages and were never a mass production item. Their relative rarity, of course, is why genuine pieces of 19th century scrimshaw are considered to be valuable collectibles today. Reference: McNamara, Robert. "Objects Made From the Whaling Industry." ThoughtCo, Jul. 31, 2021, thoughtco.com/products-produced-from-whales-1774070.Whale bone was an important commodity, used in corsets, collar stays, buggy whips, and toys.Whale bone piece. Advanced stage of calcification as indicated by deep pitting. Off white to grey.None.flagstaff hill, warrnambool, shipwrecked-coast, flagstaff-hill, flagstaff-hill-maritime-museum, maritime-museum, shipwreck-coast, flagstaff-hill-maritime-village, whales, whale bone, corsets, toys, whips -
 Flagstaff Hill Maritime Museum and Village
Flagstaff Hill Maritime Museum and VillageAnimal specimen - Whale bone, Undetermined
Prior to carrying out a detailed condition report of the cetacean skeletons, it is useful to have an understanding of the materials we are likely to encounter, in terms of structure and chemistry. This entry invites you to join in learning about the composition of whale bone and oil. Whale bone (Cetacean) bone is comprised of a composite structure of both an inorganic matrix of mainly hydroxylapatite (a calcium phosphate mineral), providing strength and rigidity, as well as an organic protein ‘scaffolding’ of mainly collagen, facilitating growth and repair (O’Connor 2008, CCI 2010). Collagen is also the structural protein component in cartilage between the whale vertebrae and attached to the fins of both the Killer Whale and the Dolphin. Relative proportions in the bone composition (affecting density), are linked with the feeding habits and mechanical stresses typically endured by bones of particular whale types. A Sperm Whale (Physeter macrocephalus Linnaeus, 1758) skeleton (toothed) thus has a higher mineral value (~67%) than a Fin Whale (Balaenoptera physalus Linnaeus, 1758) (baleen) (~60%) (Turner Walker 2012). The internal structure of bone can be divided into compact and cancellous bone. In whales, load-bearing structures such as mandibles and upper limb bones (e.g. humerus, sternum) are largely composed of compact bone (Turner Walker 2012). This consists of lamella concentrically deposited around the longitudinal axis and is permeated by fluid carrying channels (O’Connor 2008). Cancellous (spongy) bone, with a highly porous angular network of trabeculae, is less stiff and thus found in whale ribs and vertebrae (Turner Walker 2012). Whale oil Whales not only carry a thick layer of fat (blubber) in the soft tissue of their body for heat insulation and as a food store while they are alive, but also hold large oil (lipid) reserves in their porous bones. Following maceration of the whale skeleton after death to remove the soft tissue, the bones retain a high lipid content (Higgs et. al 2010). Particularly bones with a spongy (porous) structure have a high capacity to hold oil-rich marrow. Comparative data of various whale species suggests the skull, particularly the cranium and mandible bones are particularly oil rich. Along the vertebral column, the lipid content is reduced, particularly in the thoracic vertebrae (~10-25%), yet greatly increases from the lumbar to the caudal vertebrae (~40-55%). The chest area (scapula, sternum and ribs) show a mid-range lipid content (~15-30%), with vertically orientated ribs being more heavily soaked lower down (Turner Walker 2012, Higgs et. al 2010). Whale oil is largely composed of triglycerides (molecules of fatty acids attached to a glycerol molecule). In Arctic whales a higher proportion of unsaturated, versus saturated fatty acids make up the lipid. Unsaturated fatty acids (with double or triple carbon bonds causing chain kinks, preventing close packing (solidifying) of molecules), are more likely to be liquid (oil), versus solid (fat) at room temperature (Smith and March 2007). Objects Made From the Whaling Industry We all know that men set forth in sailing ships and risked their lives to harpoon whales on the open seas throughout the 1800s. And while Moby Dick and other tales have made whaling stories immortal, people today generally don't appreciate that the whalers were part of a well-organized industry. The ships that set out from ports in New England roamed as far as the Pacific in hunt of specific species of whales. Adventure may have been the draw for some whalers, but for the captains who owned whaling ships, and the investors which financed voyages, there was a considerable monetary payoff. The gigantic carcasses of whales were chopped and boiled down and turned into products such as the fine oil needed to lubricate increasing advanced machine tools. And beyond the oil derived from whales, even their bones, in an era before the invention of plastic, was used to make a wide variety of consumer goods. In short, whales were a valuable natural resource the same as wood, minerals, or petroleum we now pump from the ground. Oil From Whale’s Blubber Oil was the main product sought from whales, and it was used to lubricate machinery and to provide illumination by burning it in lamps. When a whale was killed, it was towed to the ship and its blubber, the thick insulating fat under its skin, would be peeled and cut from its carcass in a process known as “flensing.” The blubber was minced into chunks and boiled in large vats on board the whaling ship, producing oil. The oil taken from whale blubber was packaged in casks and transported back to the whaling ship’s home port (such as New Bedford, Massachusetts, the busiest American whaling port in the mid-1800s). From the ports it would be sold and transported across the country and would find its way into a huge variety of products. Whale oil, in addition to be used for lubrication and illumination, was also used to manufacture soaps, paint, and varnish. Whale oil was also utilized in some processes used to manufacture textiles and rope. Spermaceti, a Highly Regarded Oil A peculiar oil found in the head of the sperm whale, spermaceti, was highly prized. The oil was waxy, and was commonly used in making candles. In fact, candles made of spermaceti were considered the best in the world, producing a bright clear flame without an excess of smoke. Spermaceti was also used, distilled in liquid form, as an oil to fuel lamps. The main American whaling port, New Bedford, Massachusetts, was thus known as "The City That Lit the World." When John Adams was the ambassador to Great Britain before serving as president he recorded in his diary a conversation about spermaceti he had with the British Prime Minister William Pitt. Adams, keen to promote the New England whaling industry, was trying to convince the British to import spermaceti sold by American whalers, which the British could use to fuel street lamps. The British were not interested. In his diary, Adams wrote that he told Pitt, “the fat of the spermaceti whale gives the clearest and most beautiful flame of any substance that is known in nature, and we are surprised you prefer darkness, and consequent robberies, burglaries, and murders in your streets to receiving as a remittance our spermaceti oil.” Despite the failed sales pitch John Adams made in the late 1700s, the American whaling industry boomed in the early to mid-1800s. And spermaceti was a major component of that success. Spermaceti could be refined into a lubricant that was ideal for precision machinery. The machine tools that made the growth of industry possible in the United States were lubricated, and essentially made possible, by oil derived from spermaceti. Baleen, or "Whalebone" The bones and teeth of various species of whales were used in a number of products, many of them common implements in a 19th century household. Whales are said to have produced “the plastic of the 1800s.” The "bone" of the whale which was most commonly used wasn’t technically a bone, it was baleen, a hard material arrayed in large plates, like gigantic combs, in the mouths of some species of whales. The purpose of the baleen is to act as a sieve, catching tiny organisms in sea water, which the whale consumes as food. As baleen was tough yet flexible, it could be used in a number of practical applications. And it became commonly known as "whalebone." Perhaps the most common use of whalebone was in the manufacture of corsets, which fashionable ladies in the 1800s wore to compress their waistlines. One typical corset advertisement from the 1800s proudly proclaims, “Real Whalebone Only Used.” Whalebone was also used for collar stays, buggy whips, and toys. Its remarkable flexibility even caused it to be used as the springs in early typewriters. The comparison to plastic is apt. Think of common items which today might be made of plastic, and it's likely that similar items in the 1800s would have been made of whalebone. Baleen whales do not have teeth. But the teeth of other whales, such as the sperm whale, would be used as ivory in such products as chess pieces, piano keys, or the handles of walking sticks. Pieces of scrimshaw, or carved whale's teeth, would probably be the best remembered use of whale's teeth. However, the carved teeth were created to pass the time on whaling voyages and were never a mass production item. Their relative rarity, of course, is why genuine pieces of 19th century scrimshaw are considered to be valuable collectibles today. Reference: McNamara, Robert. "Objects Made From the Whaling Industry." ThoughtCo, Jul. 31, 2021, thoughtco.com/products-produced-from-whales-1774070.Whale bone was an important commodity, used in corsets, collar stays, buggy whips, and toys.Whale bone piece. Advanced stage of calcification as indicated by deep pitting. Off white to grey.None.flagstaff hill, warrnambool, shipwrecked-coast, flagstaff-hill, flagstaff-hill-maritime-museum, maritime-museum, shipwreck-coast, flagstaff-hill-maritime-village, whales, whale bone, corsets, toys, whips -
 Flagstaff Hill Maritime Museum and Village
Flagstaff Hill Maritime Museum and VillageAnimal specimen - Whale bone, Undetermined
Prior to carrying out a detailed condition report of the cetacean skeletons, it is useful to have an understanding of the materials we are likely to encounter, in terms of structure and chemistry. This entry invites you to join in learning about the composition of whale bone and oil. Whale bone (Cetacean) bone is comprised of a composite structure of both an inorganic matrix of mainly hydroxylapatite (a calcium phosphate mineral), providing strength and rigidity, as well as an organic protein ‘scaffolding’ of mainly collagen, facilitating growth and repair (O’Connor 2008, CCI 2010). Collagen is also the structural protein component in cartilage between the whale vertebrae and attached to the fins of both the Killer Whale and the Dolphin. Relative proportions in the bone composition (affecting density), are linked with the feeding habits and mechanical stresses typically endured by bones of particular whale types. A Sperm Whale (Physeter macrocephalus Linnaeus, 1758) skeleton (toothed) thus has a higher mineral value (~67%) than a Fin Whale (Balaenoptera physalus Linnaeus, 1758) (baleen) (~60%) (Turner Walker 2012). The internal structure of bone can be divided into compact and cancellous bone. In whales, load-bearing structures such as mandibles and upper limb bones (e.g. humerus, sternum) are largely composed of compact bone (Turner Walker 2012). This consists of lamella concentrically deposited around the longitudinal axis and is permeated by fluid carrying channels (O’Connor 2008). Cancellous (spongy) bone, with a highly porous angular network of trabeculae, is less stiff and thus found in whale ribs and vertebrae (Turner Walker 2012). Whale oil Whales not only carry a thick layer of fat (blubber) in the soft tissue of their body for heat insulation and as a food store while they are alive, but also hold large oil (lipid) reserves in their porous bones. Following maceration of the whale skeleton after death to remove the soft tissue, the bones retain a high lipid content (Higgs et. al 2010). Particularly bones with a spongy (porous) structure have a high capacity to hold oil-rich marrow. Comparative data of various whale species suggests the skull, particularly the cranium and mandible bones are particularly oil rich. Along the vertebral column, the lipid content is reduced, particularly in the thoracic vertebrae (~10-25%), yet greatly increases from the lumbar to the caudal vertebrae (~40-55%). The chest area (scapula, sternum and ribs) show a mid-range lipid content (~15-30%), with vertically orientated ribs being more heavily soaked lower down (Turner Walker 2012, Higgs et. al 2010). Whale oil is largely composed of triglycerides (molecules of fatty acids attached to a glycerol molecule). In Arctic whales a higher proportion of unsaturated, versus saturated fatty acids make up the lipid. Unsaturated fatty acids (with double or triple carbon bonds causing chain kinks, preventing close packing (solidifying) of molecules), are more likely to be liquid (oil), versus solid (fat) at room temperature (Smith and March 2007). Objects Made From the Whaling Industry We all know that men set forth in sailing ships and risked their lives to harpoon whales on the open seas throughout the 1800s. And while Moby Dick and other tales have made whaling stories immortal, people today generally don't appreciate that the whalers were part of a well-organized industry. The ships that set out from ports in New England roamed as far as the Pacific in hunt of specific species of whales. Adventure may have been the draw for some whalers, but for the captains who owned whaling ships, and the investors which financed voyages, there was a considerable monetary payoff. The gigantic carcasses of whales were chopped and boiled down and turned into products such as the fine oil needed to lubricate increasing advanced machine tools. And beyond the oil derived from whales, even their bones, in an era before the invention of plastic, was used to make a wide variety of consumer goods. In short, whales were a valuable natural resource the same as wood, minerals, or petroleum we now pump from the ground. Oil From Whale’s Blubber Oil was the main product sought from whales, and it was used to lubricate machinery and to provide illumination by burning it in lamps. When a whale was killed, it was towed to the ship and its blubber, the thick insulating fat under its skin, would be peeled and cut from its carcass in a process known as “flensing.” The blubber was minced into chunks and boiled in large vats on board the whaling ship, producing oil. The oil taken from whale blubber was packaged in casks and transported back to the whaling ship’s home port (such as New Bedford, Massachusetts, the busiest American whaling port in the mid-1800s). From the ports it would be sold and transported across the country and would find its way into a huge variety of products. Whale oil, in addition to be used for lubrication and illumination, was also used to manufacture soaps, paint, and varnish. Whale oil was also utilized in some processes used to manufacture textiles and rope. Spermaceti, a Highly Regarded Oil A peculiar oil found in the head of the sperm whale, spermaceti, was highly prized. The oil was waxy, and was commonly used in making candles. In fact, candles made of spermaceti were considered the best in the world, producing a bright clear flame without an excess of smoke. Spermaceti was also used, distilled in liquid form, as an oil to fuel lamps. The main American whaling port, New Bedford, Massachusetts, was thus known as "The City That Lit the World." When John Adams was the ambassador to Great Britain before serving as president he recorded in his diary a conversation about spermaceti he had with the British Prime Minister William Pitt. Adams, keen to promote the New England whaling industry, was trying to convince the British to import spermaceti sold by American whalers, which the British could use to fuel street lamps. The British were not interested. In his diary, Adams wrote that he told Pitt, “the fat of the spermaceti whale gives the clearest and most beautiful flame of any substance that is known in nature, and we are surprised you prefer darkness, and consequent robberies, burglaries, and murders in your streets to receiving as a remittance our spermaceti oil.” Despite the failed sales pitch John Adams made in the late 1700s, the American whaling industry boomed in the early to mid-1800s. And spermaceti was a major component of that success. Spermaceti could be refined into a lubricant that was ideal for precision machinery. The machine tools that made the growth of industry possible in the United States were lubricated, and essentially made possible, by oil derived from spermaceti. Baleen, or "Whalebone" The bones and teeth of various species of whales were used in a number of products, many of them common implements in a 19th century household. Whales are said to have produced “the plastic of the 1800s.” The "bone" of the whale which was most commonly used wasn’t technically a bone, it was baleen, a hard material arrayed in large plates, like gigantic combs, in the mouths of some species of whales. The purpose of the baleen is to act as a sieve, catching tiny organisms in sea water, which the whale consumes as food. As baleen was tough yet flexible, it could be used in a number of practical applications. And it became commonly known as "whalebone." Perhaps the most common use of whalebone was in the manufacture of corsets, which fashionable ladies in the 1800s wore to compress their waistlines. One typical corset advertisement from the 1800s proudly proclaims, “Real Whalebone Only Used.” Whalebone was also used for collar stays, buggy whips, and toys. Its remarkable flexibility even caused it to be used as the springs in early typewriters. The comparison to plastic is apt. Think of common items which today might be made of plastic, and it's likely that similar items in the 1800s would have been made of whalebone. Baleen whales do not have teeth. But the teeth of other whales, such as the sperm whale, would be used as ivory in such products as chess pieces, piano keys, or the handles of walking sticks. Pieces of scrimshaw, or carved whale's teeth, would probably be the best remembered use of whale's teeth. However, the carved teeth were created to pass the time on whaling voyages and were never a mass production item. Their relative rarity, of course, is why genuine pieces of 19th century scrimshaw are considered to be valuable collectibles today. Reference: McNamara, Robert. "Objects Made From the Whaling Industry." ThoughtCo, Jul. 31, 2021, thoughtco.com/products-produced-from-whales-1774070.Whale bone was an important commodity, used in corsets, collar stays, buggy whips, and toys.Whale bone vertebrae. Advanced stage of calcification as indicated by deep pitting. Off white to grey.Noneflagstaff hill, warrnambool, shipwrecked-coast, flagstaff-hill, flagstaff-hill-maritime-museum, maritime-museum, shipwreck-coast, flagstaff-hill-maritime-village, whales, whale bone, corsets, toys, whips, whalebone -
 Flagstaff Hill Maritime Museum and Village
Flagstaff Hill Maritime Museum and VillageAnimal specimen - Whale Vertebrae, Undetermined
Prior to carrying out a detailed condition report of the cetacean skeletons, it is useful to have an understanding of the materials we are likely to encounter, in terms of structure and chemistry. This entry invites you to join in learning about the composition of whale bone and oil. Whale bone (Cetacean) bone is comprised of a composite structure of both an inorganic matrix of mainly hydroxylapatite (a calcium phosphate mineral), providing strength and rigidity, as well as an organic protein ‘scaffolding’ of mainly collagen, facilitating growth and repair (O’Connor 2008, CCI 2010). Collagen is also the structural protein component in cartilage between the whale vertebrae and attached to the fins of both the Killer Whale and the Dolphin. Relative proportions in the bone composition (affecting density), are linked with the feeding habits and mechanical stresses typically endured by bones of particular whale types. A Sperm Whale (Physeter macrocephalus Linnaeus, 1758) skeleton (toothed) thus has a higher mineral value (~67%) than a Fin Whale (Balaenoptera physalus Linnaeus, 1758) (baleen) (~60%) (Turner Walker 2012). The internal structure of bone can be divided into compact and cancellous bone. In whales, load-bearing structures such as mandibles and upper limb bones (e.g. humerus, sternum) are largely composed of compact bone (Turner Walker 2012). This consists of lamella concentrically deposited around the longitudinal axis and is permeated by fluid carrying channels (O’Connor 2008). Cancellous (spongy) bone, with a highly porous angular network of trabeculae, is less stiff and thus found in whale ribs and vertebrae (Turner Walker 2012). Whale oil Whales not only carry a thick layer of fat (blubber) in the soft tissue of their body for heat insulation and as a food store while they are alive, but also hold large oil (lipid) reserves in their porous bones. Following maceration of the whale skeleton after death to remove the soft tissue, the bones retain a high lipid content (Higgs et. al 2010). Particularly bones with a spongy (porous) structure have a high capacity to hold oil-rich marrow. Comparative data of various whale species suggests the skull, particularly the cranium and mandible bones are particularly oil rich. Along the vertebral column, the lipid content is reduced, particularly in the thoracic vertebrae (~10-25%), yet greatly increases from the lumbar to the caudal vertebrae (~40-55%). The chest area (scapula, sternum and ribs) show a mid-range lipid content (~15-30%), with vertically orientated ribs being more heavily soaked lower down (Turner Walker 2012, Higgs et. al 2010). Whale oil is largely composed of triglycerides (molecules of fatty acids attached to a glycerol molecule). In Arctic whales a higher proportion of unsaturated, versus saturated fatty acids make up the lipid. Unsaturated fatty acids (with double or triple carbon bonds causing chain kinks, preventing close packing (solidifying) of molecules), are more likely to be liquid (oil), versus solid (fat) at room temperature (Smith and March 2007). Objects Made From the Whaling Industry We all know that men set forth in sailing ships and risked their lives to harpoon whales on the open seas throughout the 1800s. And while Moby Dick and other tales have made whaling stories immortal, people today generally don't appreciate that the whalers were part of a well-organized industry. The ships that set out from ports in New England roamed as far as the Pacific in hunt of specific species of whales. Adventure may have been the draw for some whalers, but for the captains who owned whaling ships, and the investors which financed voyages, there was a considerable monetary payoff. The gigantic carcasses of whales were chopped and boiled down and turned into products such as the fine oil needed to lubricate increasing advanced machine tools. And beyond the oil derived from whales, even their bones, in an era before the invention of plastic, was used to make a wide variety of consumer goods. In short, whales were a valuable natural resource the same as wood, minerals, or petroleum we now pump from the ground. Oil From Whale’s Blubber Oil was the main product sought from whales, and it was used to lubricate machinery and to provide illumination by burning it in lamps. When a whale was killed, it was towed to the ship and its blubber, the thick insulating fat under its skin, would be peeled and cut from its carcass in a process known as “flensing.” The blubber was minced into chunks and boiled in large vats on board the whaling ship, producing oil. The oil taken from whale blubber was packaged in casks and transported back to the whaling ship’s home port (such as New Bedford, Massachusetts, the busiest American whaling port in the mid-1800s). From the ports it would be sold and transported across the country and would find its way into a huge variety of products. Whale oil, in addition to be used for lubrication and illumination, was also used to manufacture soaps, paint, and varnish. Whale oil was also utilized in some processes used to manufacture textiles and rope. Spermaceti, a Highly Regarded Oil A peculiar oil found in the head of the sperm whale, spermaceti, was highly prized. The oil was waxy, and was commonly used in making candles. In fact, candles made of spermaceti were considered the best in the world, producing a bright clear flame without an excess of smoke. Spermaceti was also used, distilled in liquid form, as an oil to fuel lamps. The main American whaling port, New Bedford, Massachusetts, was thus known as "The City That Lit the World." When John Adams was the ambassador to Great Britain before serving as president he recorded in his diary a conversation about spermaceti he had with the British Prime Minister William Pitt. Adams, keen to promote the New England whaling industry, was trying to convince the British to import spermaceti sold by American whalers, which the British could use to fuel street lamps. The British were not interested. In his diary, Adams wrote that he told Pitt, “the fat of the spermaceti whale gives the clearest and most beautiful flame of any substance that is known in nature, and we are surprised you prefer darkness, and consequent robberies, burglaries, and murders in your streets to receiving as a remittance our spermaceti oil.” Despite the failed sales pitch John Adams made in the late 1700s, the American whaling industry boomed in the early to mid-1800s. And spermaceti was a major component of that success. Spermaceti could be refined into a lubricant that was ideal for precision machinery. The machine tools that made the growth of industry possible in the United States were lubricated, and essentially made possible, by oil derived from spermaceti. Whalebone The bones and teeth of various species of whales were used in a number of products, many of them common implements in a 19th century household. Whales are said to have produced “the plastic of the 1800s.” The bone of the whale which was most commonly used wasn’t technically a bone, it was baleen, a hard material arrayed in large plates, like gigantic combs, in the mouths of some species of whales. The purpose of the baleen is to act as a sieve, catching tiny organisms in sea water, which the whale consumes as food. As baleen was tough yet flexible, it could be used in a number of practical applications. And it became commonly known as whalebone. Perhaps the most common use of whalebone was in the manufacture of corsets, which fashionable ladies in the 1800s wore to compress their waistlines. One typical corset advertisement from the 1800s proudly proclaims, “Real Whalebone Only Used.” Whalebone was also used for collar stays, buggy whips, and toys. Its remarkable flexibility even caused it to be used as the springs in early typewriters. The comparison to plastic is apt. Think of common items which today might be made of plastic, and it's likely that similar items in the 1800s would have been made of whalebone. Baleen whales do not have teeth. But the teeth of other whales, such as the sperm whale, would be used as ivory in such products as chess pieces, piano keys, or the handles of walking sticks. Pieces of scrimshaw, or carved whale's teeth, would probably be the best remembered use of whale's teeth. However, the carved teeth were created to pass the time on whaling voyages and were never a mass production item. Their relative rarity, of course, is why genuine pieces of 19th century scrimshaw are considered to be valuable collectibles today. Reference: McNamara, Robert. "Objects Made From the Whaling Industry." ThoughtCo, Jul. 31, 2021, thoughtco.com/products-produced-from-whales-1774070.Whale bone during the 17th, 18th, 19th and early 20th centuries was an important industry providing an important commodity. Whales from these times provided everything from lighting & machine oils to using the animal's bones for use in corsets, collar stays, buggy whips, and many other everyday items then in use.Whale bone Vertebrae with advanced stage of calcification as indicated by deep pitting. Off white to grey.None.warrnambool, flagstaff-hill, flagstaff-hill-maritime-museum, maritime-museum, shipwreck-coast, flagstaff-hill-maritime-village, whale bones, whale skeleton, whales, whale bone, corsets, toys, whips, whaleling industry, maritime fishing, whalebone -
 Flagstaff Hill Maritime Museum and Village
Flagstaff Hill Maritime Museum and VillageAnimal specimen - Whale Jaw Bone, Undetermined
Prior to carrying out a detailed condition report of the cetacean skeletons, it is useful to have an understanding of the materials we are likely to encounter, in terms of structure and chemistry. This entry invites you to join in learning about the composition of whale bone and oil. Whale bone (Cetacean) bone is comprised of a composite structure of both an inorganic matrix of mainly hydroxylapatite (a calcium phosphate mineral), providing strength and rigidity, as well as an organic protein ‘scaffolding’ of mainly collagen, facilitating growth and repair (O’Connor 2008, CCI 2010). Collagen is also the structural protein component in cartilage between the whale vertebrae and attached to the fins of both the Killer Whale and the Dolphin. Relative proportions in the bone composition (affecting density), are linked with the feeding habits and mechanical stresses typically endured by bones of particular whale types. A Sperm Whale (Physeter macrocephalus Linnaeus, 1758) skeleton (toothed) thus has a higher mineral value (~67%) than a Fin Whale (Balaenoptera physalus Linnaeus, 1758) (baleen) (~60%) (Turner Walker 2012). The internal structure of bone can be divided into compact and cancellous bone. In whales, load-bearing structures such as mandibles and upper limb bones (e.g. humerus, sternum) are largely composed of compact bone (Turner Walker 2012). This consists of lamella concentrically deposited around the longitudinal axis and is permeated by fluid carrying channels (O’Connor 2008). Cancellous (spongy) bone, with a highly porous angular network of trabeculae, is less stiff and thus found in whale ribs and vertebrae (Turner Walker 2012). Whale oil Whales not only carry a thick layer of fat (blubber) in the soft tissue of their body for heat insulation and as a food store while they are alive, but also hold large oil (lipid) reserves in their porous bones. Following maceration of the whale skeleton after death to remove the soft tissue, the bones retain a high lipid content (Higgs et. al 2010). Particularly bones with a spongy (porous) structure have a high capacity to hold oil-rich marrow. Comparative data of various whale species suggests the skull, particularly the cranium and mandible bones are particularly oil rich. Along the vertebral column, the lipid content is reduced, particularly in the thoracic vertebrae (~10-25%), yet greatly increases from the lumbar to the caudal vertebrae (~40-55%). The chest area (scapula, sternum and ribs) show a mid-range lipid content (~15-30%), with vertically orientated ribs being more heavily soaked lower down (Turner Walker 2012, Higgs et. al 2010). Whale oil is largely composed of triglycerides (molecules of fatty acids attached to a glycerol molecule). In Arctic whales a higher proportion of unsaturated, versus saturated fatty acids make up the lipid. Unsaturated fatty acids (with double or triple carbon bonds causing chain kinks, preventing close packing (solidifying) of molecules), are more likely to be liquid (oil), versus solid (fat) at room temperature (Smith and March 2007). Objects Made From the Whaling Industry We all know that men set forth in sailing ships and risked their lives to harpoon whales on the open seas throughout the 1800s. And while Moby Dick and other tales have made whaling stories immortal, people today generally don't appreciate that the whalers were part of a well-organized industry. The ships that set out from ports in New England roamed as far as the Pacific in hunt of specific species of whales. Adventure may have been the draw for some whalers, but for the captains who owned whaling ships, and the investors which financed voyages, there was a considerable monetary payoff. The gigantic carcasses of whales were chopped and boiled down and turned into products such as the fine oil needed to lubricate increasing advanced machine tools. And beyond the oil derived from whales, even their bones, in an era before the invention of plastic, was used to make a wide variety of consumer goods. In short, whales were a valuable natural resource the same as wood, minerals, or petroleum we now pump from the ground. Oil From Whale’s Blubber Oil was the main product sought from whales, and it was used to lubricate machinery and to provide illumination by burning it in lamps. When a whale was killed, it was towed to the ship and its blubber, the thick insulating fat under its skin, would be peeled and cut from its carcass in a process known as “flensing.” The blubber was minced into chunks and boiled in large vats on board the whaling ship, producing oil. The oil taken from whale blubber was packaged in casks and transported back to the whaling ship’s home port (such as New Bedford, Massachusetts, the busiest American whaling port in the mid-1800s). From the ports it would be sold and transported across the country and would find its way into a huge variety of products. Whale oil, in addition to be used for lubrication and illumination, was also used to manufacture soaps, paint, and varnish. Whale oil was also utilized in some processes used to manufacture textiles and rope. Spermaceti, a Highly Regarded Oil A peculiar oil found in the head of the sperm whale, spermaceti, was highly prized. The oil was waxy, and was commonly used in making candles. In fact, candles made of spermaceti were considered the best in the world, producing a bright clear flame without an excess of smoke. Spermaceti was also used, distilled in liquid form, as an oil to fuel lamps. The main American whaling port, New Bedford, Massachusetts, was thus known as "The City That Lit the World." When John Adams was the ambassador to Great Britain before serving as president he recorded in his diary a conversation about spermaceti he had with the British Prime Minister William Pitt. Adams, keen to promote the New England whaling industry, was trying to convince the British to import spermaceti sold by American whalers, which the British could use to fuel street lamps. The British were not interested. In his diary, Adams wrote that he told Pitt, “the fat of the spermaceti whale gives the clearest and most beautiful flame of any substance that is known in nature, and we are surprised you prefer darkness, and consequent robberies, burglaries, and murders in your streets to receiving as a remittance our spermaceti oil.” Despite the failed sales pitch John Adams made in the late 1700s, the American whaling industry boomed in the early to mid-1800s. And spermaceti was a major component of that success. Spermaceti could be refined into a lubricant that was ideal for precision machinery. The machine tools that made the growth of industry possible in the United States were lubricated, and essentially made possible, by oil derived from spermaceti. Baleen, or "Whalebone" The bones and teeth of various species of whales were used in a number of products, many of them common implements in a 19th century household. Whales are said to have produced “the plastic of the 1800s.” The "bone" of the whale which was most commonly used wasn’t technically a bone, it was baleen, a hard material arrayed in large plates, like gigantic combs, in the mouths of some species of whales. The purpose of the baleen is to act as a sieve, catching tiny organisms in sea water, which the whale consumes as food. As baleen was tough yet flexible, it could be used in a number of practical applications. And it became commonly known as "whalebone." Perhaps the most common use of whalebone was in the manufacture of corsets, which fashionable ladies in the 1800s wore to compress their waistlines. One typical corset advertisement from the 1800s proudly proclaims, “Real Whalebone Only Used.” Whalebone was also used for collar stays, buggy whips, and toys. Its remarkable flexibility even caused it to be used as the springs in early typewriters. The comparison to plastic is apt. Think of common items which today might be made of plastic, and it's likely that similar items in the 1800s would have been made of whalebone. Baleen whales do not have teeth. But the teeth of other whales, such as the sperm whale, would be used as ivory in such products as chess pieces, piano keys, or the handles of walking sticks. Pieces of scrimshaw, or carved whale's teeth, would probably be the best remembered use of whale's teeth. However, the carved teeth were created to pass the time on whaling voyages and were never a mass production item. Their relative rarity, of course, is why genuine pieces of 19th century scrimshaw are considered to be valuable collectibles today. Reference: McNamara, Robert. "Objects Made From the Whaling Industry." ThoughtCo, Jul. 31, 2021, thoughtco.com/products-produced-from-whales-1774070.Whale bone during the 17th, 18th, 19th and early 20th centuries was an important industry providing an important commodity. Whales from these times provided everything from lighting & machine oils to using the animal's bones for use in corsets, collar stays, buggy whips, and many other everyday items then in use.Whale jaw bone one side, long & curved with advanced stage of calcification off white to grey.None.warrnambool, flagstaff-hill, flagstaff-hill-maritime-museum, maritime-museum, shipwreck-coast, flagstaff-hill-maritime-village, whale bones, whale skeleton, whales, whale bone, corsets, toys, whips, whaleling industry, maritime fishing, whalebone -
 Flagstaff Hill Maritime Museum and Village
Flagstaff Hill Maritime Museum and VillageAnimal specimen - Whale Rib Bone, Undetermined
Prior to carrying out a detailed condition report of the cetacean skeletons, it is useful to have an understanding of the materials we are likely to encounter, in terms of structure and chemistry. This entry invites you to join in learning about the composition of whale bone and oil. Whale bone (Cetacean) bone is comprised of a composite structure of both an inorganic matrix of mainly hydroxylapatite (a calcium phosphate mineral), providing strength and rigidity, as well as an organic protein ‘scaffolding’ of mainly collagen, facilitating growth and repair (O’Connor 2008, CCI 2010). Collagen is also the structural protein component in cartilage between the whale vertebrae and attached to the fins of both the Killer Whale and the Dolphin. Relative proportions in the bone composition (affecting density), are linked with the feeding habits and mechanical stresses typically endured by bones of particular whale types. A Sperm Whale (Physeter macrocephalus Linnaeus, 1758) skeleton (toothed) thus has a higher mineral value (~67%) than a Fin Whale (Balaenoptera physalus Linnaeus, 1758) (baleen) (~60%) (Turner Walker 2012). The internal structure of bone can be divided into compact and cancellous bone. In whales, load-bearing structures such as mandibles and upper limb bones (e.g. humerus, sternum) are largely composed of compact bone (Turner Walker 2012). This consists of lamella concentrically deposited around the longitudinal axis and is permeated by fluid carrying channels (O’Connor 2008). Cancellous (spongy) bone, with a highly porous angular network of trabeculae, is less stiff and thus found in whale ribs and vertebrae (Turner Walker 2012). Whale oil Whales not only carry a thick layer of fat (blubber) in the soft tissue of their body for heat insulation and as a food store while they are alive, but also hold large oil (lipid) reserves in their porous bones. Following maceration of the whale skeleton after death to remove the soft tissue, the bones retain a high lipid content (Higgs et. al 2010). Particularly bones with a spongy (porous) structure have a high capacity to hold oil-rich marrow. Comparative data of various whale species suggests the skull, particularly the cranium and mandible bones are particularly oil rich. Along the vertebral column, the lipid content is reduced, particularly in the thoracic vertebrae (~10-25%), yet greatly increases from the lumbar to the caudal vertebrae (~40-55%). The chest area (scapula, sternum and ribs) show a mid-range lipid content (~15-30%), with vertically orientated ribs being more heavily soaked lower down (Turner Walker 2012, Higgs et. al 2010). Whale oil is largely composed of triglycerides (molecules of fatty acids attached to a glycerol molecule). In Arctic whales a higher proportion of unsaturated, versus saturated fatty acids make up the lipid. Unsaturated fatty acids (with double or triple carbon bonds causing chain kinks, preventing close packing (solidifying) of molecules), are more likely to be liquid (oil), versus solid (fat) at room temperature (Smith and March 2007). Objects Made From the Whaling Industry We all know that men set forth in sailing ships and risked their lives to harpoon whales on the open seas throughout the 1800s. And while Moby Dick and other tales have made whaling stories immortal, people today generally don't appreciate that the whalers were part of a well-organized industry. The ships that set out from ports in New England roamed as far as the Pacific in hunt of specific species of whales. Adventure may have been the draw for some whalers, but for the captains who owned whaling ships, and the investors which financed voyages, there was a considerable monetary payoff. The gigantic carcasses of whales were chopped and boiled down and turned into products such as the fine oil needed to lubricate increasing advanced machine tools. And beyond the oil derived from whales, even their bones, in an era before the invention of plastic, was used to make a wide variety of consumer goods. In short, whales were a valuable natural resource the same as wood, minerals, or petroleum we now pump from the ground. Oil From Whale’s Blubber Oil was the main product sought from whales, and it was used to lubricate machinery and to provide illumination by burning it in lamps. When a whale was killed, it was towed to the ship and its blubber, the thick insulating fat under its skin, would be peeled and cut from its carcass in a process known as “flensing.” The blubber was minced into chunks and boiled in large vats on board the whaling ship, producing oil. The oil taken from whale blubber was packaged in casks and transported back to the whaling ship’s home port (such as New Bedford, Massachusetts, the busiest American whaling port in the mid-1800s). From the ports it would be sold and transported across the country and would find its way into a huge variety of products. Whale oil, in addition to be used for lubrication and illumination, was also used to manufacture soaps, paint, and varnish. Whale oil was also utilized in some processes used to manufacture textiles and rope. Spermaceti, a Highly Regarded Oil A peculiar oil found in the head of the sperm whale, spermaceti, was highly prized. The oil was waxy, and was commonly used in making candles. In fact, candles made of spermaceti were considered the best in the world, producing a bright clear flame without an excess of smoke. Spermaceti was also used, distilled in liquid form, as an oil to fuel lamps. The main American whaling port, New Bedford, Massachusetts, was thus known as "The City That Lit the World." When John Adams was the ambassador to Great Britain before serving as president he recorded in his diary a conversation about spermaceti he had with the British Prime Minister William Pitt. Adams, keen to promote the New England whaling industry, was trying to convince the British to import spermaceti sold by American whalers, which the British could use to fuel street lamps. The British were not interested. In his diary, Adams wrote that he told Pitt, “the fat of the spermaceti whale gives the clearest and most beautiful flame of any substance that is known in nature, and we are surprised you prefer darkness, and consequent robberies, burglaries, and murders in your streets to receiving as a remittance our spermaceti oil.” Despite the failed sales pitch John Adams made in the late 1700s, the American whaling industry boomed in the early to mid-1800s. And spermaceti was a major component of that success. Spermaceti could be refined into a lubricant that was ideal for precision machinery. The machine tools that made the growth of industry possible in the United States were lubricated, and essentially made possible, by oil derived from spermaceti. Baleen, or "Whalebone" The bones and teeth of various species of whales were used in a number of products, many of them common implements in a 19th century household. Whales are said to have produced “the plastic of the 1800s.” The "bone" of the whale which was most commonly used wasn’t technically a bone, it was baleen, a hard material arrayed in large plates, like gigantic combs, in the mouths of some species of whales. The purpose of the baleen is to act as a sieve, catching tiny organisms in sea water, which the whale consumes as food. As baleen was tough yet flexible, it could be used in a number of practical applications. And it became commonly known as "whalebone." Perhaps the most common use of whalebone was in the manufacture of corsets, which fashionable ladies in the 1800s wore to compress their waistlines. One typical corset advertisement from the 1800s proudly proclaims, “Real Whalebone Only Used.” Whalebone was also used for collar stays, buggy whips, and toys. Its remarkable flexibility even caused it to be used as the springs in early typewriters. The comparison to plastic is apt. Think of common items which today might be made of plastic, and it's likely that similar items in the 1800s would have been made of whalebone. Baleen whales do not have teeth. But the teeth of other whales, such as the sperm whale, would be used as ivory in such products as chess pieces, piano keys, or the handles of walking sticks. Pieces of scrimshaw, or carved whale's teeth, would probably be the best remembered use of whale's teeth. However, the carved teeth were created to pass the time on whaling voyages and were never a mass production item. Their relative rarity, of course, is why genuine pieces of 19th century scrimshaw are considered to be valuable collectibles today. Reference: McNamara, Robert. "Objects Made From the Whaling Industry." ThoughtCo, Jul. 31, 2021, thoughtco.com/products-produced-from-whales-1774070.Whale bone during the 17th, 18th, 19th and early 20th centuries was an important industry providing an important commodity. Whales from these times provided everything from lighting & machine oils to using the animal's bones for use in corsets, collar stays, buggy whips, and many other everyday items then in use.Whale rib bone with advanced stage of calcification as indicated by brittleness. None.warrnambool, flagstaff-hill, flagstaff-hill-maritime-museum, maritime-museum, shipwreck-coast, flagstaff-hill-maritime-village, whale bones, whale skeleton, whales, whale bone, corsets, toys, whips, whaleling industry, maritime fishing, whalebone -
 Flagstaff Hill Maritime Museum and Village
Flagstaff Hill Maritime Museum and VillageDomestic object - Bowl, Late 19th or early 20th Century
The Process of Making Pottery Decorating, Firing, Glazing, Making, Technical There is a rhythm and flow to clay. It can’t be done all at once! Even the making process! It can take weeks to get everything done, especially if you can only work on your pottery once a week! Even though we have three hour classes, it’s often just not enough time! Here is an overview of some of the processes so you have a bit more grasp on some of the technical stuff! Step One – Design There are SO many ideas out there for making stuff in clay! From delicate porcelain jewellery, through to heavy sculptural work and everything in between. Deciding your direction is sometimes not that easy – when you first start, try everything, you will naturally gravitate to the style that you enjoy! The options and variations are endless and can get a wee bit overwhelming too! Check in with me before you start to ensure your ideas will work, what order you might do things, how you could achieve the look you are seeking and any other technical data required! Step Two – Making Clay is thixotropic. This means that as you work with it, the clay first gets sloppier and wetter, before is begins to dry in the atmosphere. For most things, you simply can’t do all parts of the project at once. An example of work order might look like: Get last weeks work out from the shelves Prepare clay for today’s work – roll your clay, prepare balls for throwing, make the first stage of a pinch pot) Clean up last week’s work and put it on the shelf for bisque firing Check that you have any glazing to do – and do enough of it that you will have time to finish your main project Do the next step of your next project – there might be a further step that can’t be complete immediately, in that case, wrap your work well and put onto the shelves. Letting your work rest for a while can really help keep your work clean and professional looking. Many things require bagging under plastic to keep it ready for work the next week – put your name on the outside of the bag so you can find your work easily. We have stickers and markers. Consider how you want to decorate your work – coloured slip can be applied at a fairly wet stage (remembering that it will make your work even wetter!). Trying to apply slip to dry clay won’t work! If you want to do sgraffito – you will need to keep the work leather hard (a state of dryness where you can still work the clay with a little effort and a little water and care). Step Three – Drying Most of the time your work can go into the rack uncovered to let it dry out for the following week. If you want to continue forming or shaping you will need to double bag your work – put your work on a suitable sized bat and put the bat in a bag so the base of the bag is under the bat, then put another bag over the top of the work and tuck the top of the bag under the bat. If you want to trim (or turn) your thrown work the following week, it should also be double bagged. If your work is large, delicate, or of uneven thicknesses, you should lightly cover your work for drying. When considering the drying process, bare in mind the weather, humidity and wind! The hotter and dryer, the faster things dry and work can dry unevenly in the shelves – this can lead to cracking – another time to lightly cover your work for drying. Step Four – Trimming and Cleaning Up Your work is dry! It is called greenware now and it is at it’s most fragile! Handle everything with two hands. I often refer to soft hands – keep everything gentle and with your fingers spread as much as possible. Try to not pick up things like plates too much, and always with both hands! Before your work can be bisque fired it should be “cleaned up”. You work won’t go into the kiln if it has sharp edges – when glazed, sharp edges turn into razor blades! Use a piece of fly wire to rub the work all over – this will scratch a little so be light handed. Use a knife or metal kidney to scrape any areas that require a bit more dynamic treatment than the fly wire offers! Finally, a very light wipe over with a slightly damp sponge can help soften and soothe all of your edges and dags! Trimming thrown work: If you are planning to trim (or turn) your thrown work (and you should be), make sure you bag it well – your work should be leather hard to almost dry for easiest trimming. Use this step to finish the work completely – use a metal kidney to polish the surface, or a slightly damp sponge to give a freshly thrown look. Wipe the sponge around the rim after trimming, and check the inside of the pot for dags! Trimming slip cast work: Usually I will trim the rims of your work on the wheel the following day to make that stage easier, however you will still need to check your work for lumps and bumps. Last but not least – check that your name is still clearly on the bottom of your work. Step Five – Bisque Firing When the work is completely dry it can go into the bisque kiln. The bisque kiln is fired to 1000°C. This process burns off the water in the clay as well as some of the chemically bound water. The structure of the clay is not altered that much at this temperature. Inside the bisque kiln, the work is stacked a little, small bowl inside a larger bowl and onto a heavy plate. Smaller items like decorations or drink coasters might get stacked several high. Consideration is paid to the weight of the stack and shape of the work. A bisque kiln can fire about one and a half times the amount of work that the glaze kiln can fire. The firing takes about 10 hours to complete the cycle and about two days to cool down. Once it has been emptied the work is placed in the glaze room ready for you to decorate! Step Six – Glazing Decorating your work with colour can be a lot of fun – and time consuming! There are three main options for surface treatment at this stage: Oxide Washes Underglazes Glazes Washes and underglazes do not “glaze” the work – It will still need a layer of glaze to fully seal the clay (washes don’t need glaze on surfaces not designed for food or liquid as they can gloss up a little on their own). Underglazes are stable colourants that turn out pretty much how they look in the jar. They can be mixed with each other to form other colours and can be used like water colours to paint onto your work. Mostly they should have a clear glaze on top to seal them. Oxides are a different species – the pink oxide (cobalt) wash turns out bright blue for instance. They don’t always need a glaze on top, and some glazes can change the colour of the wash! The glazes need no other “glaze” on top! Be careful of unknown glaze interactions – you can put any combination of glaze in a bowl or on a plate, but only a single glaze on the outside of any vertical surface! Glazes are a chemical reaction under heat. We don’t know the exact chemicals in the Mayco glazes we use. I can guess by the way they interact with each other, however, on the whole, you need to test every idea you have, and not run the test on a vertical surface! Simply put, glaze is a layer of glass like substance that bonds with the clay underneath. Clay is made of silica, alumina and water. Glaze is made of mostly silica. Silica has a melting point of 1700°C and we fire to 1240°C. The silica requires a “flux” to help it melt at the lower temperature. Fluxes can be all sorts of chemicals – a common one is calcium – calcium has a melting point of 2500°C, however, together they both melt at a much lower temperature! Colourants are metal oxides like cobalt (blue), chrome (green through black), copper (green, blue, even red!), manganese (black, purple and pink) iron (red brown), etc. Different chemicals in the glaze can have dramatic effects. for example, barium carbonate (which we don’t use) turns manganese bright pink! Other elements can turn manganese dioxide brown, blue, purple and reddish brown. Manganese dioxide is a flux in and of itself as well. So, glazes that get their black and purple colours, often interact with other glazes and RUN! Our mirror black is a good example – it mixes really well with many glazes because it fluxes them – causes them to melt faster. It will also bring out many beautiful colours in the glazes because it’s black colouring most definitely comes from manganese dioxide! Glaze chemistry is a whole subject on it’s own! We use commercial Mayco glazes on purpose – for their huge range of colour possibilities, stability, cool interactions, artistic freedom with the ability to easily brush the glazes on and ease of use. We currently have almost 50 glazes on hand! A major project is to test the interactions of all glazes with each other. That is 2,500 test tiles!!!! I’m going to make the wall behind the wheels the feature wall of pretty colours! Step Seven – Glaze (Gloss or sometimes called “Glost”) Firing Most of the time this is the final stage of making your creation (but not always!) The glaze kiln goes to 1240°C. This is called cone 6, or midrange. It is the low end of stoneware temperatures. Stoneware clays and glazes are typically fired at cone 8 – 10, that is 1260 – 1290°C. The energy requirement to go from 1240°C to 1280°C is almost a 30% more! Our clay is formulated to vitrify (mature, turn “glass-like”) at 1240°, as are our glazes. A glaze kiln take around 12 hours to reach temperature and two to three days to cool down. Sometimes a third firing process is required – this is for decoration that is added to work after the glaze firing. For example – adding precious metals and lustres. this firing temperature is usually around 600 – 800°C depending upon the techniques being used. There are many students interested in gold and silver trims – we will be doing this third type of firing soon! After firing your work will be in the student finished work shelves. Remember to pay for it before you head out the door! There is a small extra charge for using porcelain clay (it’s more than twice the price of regular clay), and for any third firing process! Once your work has been fired it can not turn back into clay for millennia – so don’t fire it if you don’t like it! Put it in the bucket for recycling. https://firebirdstudios.com.au/the-process-of-making-pottery/ The bowl is an example of kitchenware used in the 19th century and still in use today.Bowl white ceramic. Crack on side. Badly stained.Backstamp very faint and unable to be read.flagstaff hill, warrnambool, shipwrecked-coast, flagstaff-hill, flagstaff-hill-maritime-museum, maritime-museum, shipwreck-coast, flagstaff-hill-maritime-village, mixing bowl, food preparation, kitchen equipment, ceramic -
 Flagstaff Hill Maritime Museum and Village
Flagstaff Hill Maritime Museum and VillageDomestic object - Bowl
The Process of Making Pottery Decorating, Firing, Glazing, Making, Technical There is a rhythm and flow to clay. It can’t be done all at once! Even the making process! It can take weeks to get everything done, especially if you can only work on your pottery once a week! Even though we have three hour classes, it’s often just not enough time! Here is an overview of some of the processes so you have a bit more grasp on some of the technical stuff! Step One – Design There are SO many ideas out there for making stuff in clay! From delicate porcelain jewellery, through to heavy sculptural work and everything in between. Deciding your direction is sometimes not that easy – when you first start, try everything, you will naturally gravitate to the style that you enjoy! The options and variations are endless and can get a wee bit overwhelming too! Check in with me before you start to ensure your ideas will work, what order you might do things, how you could achieve the look you are seeking and any other technical data required! Step Two – Making Clay is thixotropic. This means that as you work with it, the clay first gets sloppier and wetter, before is begins to dry in the atmosphere. For most things, you simply can’t do all parts of the project at once. An example of work order might look like: Get last weeks work out from the shelves Prepare clay for today’s work – roll your clay, prepare balls for throwing, make the first stage of a pinch pot) Clean up last week’s work and put it on the shelf for bisque firing Check that you have any glazing to do – and do enough of it that you will have time to finish your main project Do the next step of your next project – there might be a further step that can’t be complete immediately, in that case, wrap your work well and put onto the shelves. Letting your work rest for a while can really help keep your work clean and professional looking. Many things require bagging under plastic to keep it ready for work the next week – put your name on the outside of the bag so you can find your work easily. We have stickers and markers. Consider how you want to decorate your work – coloured slip can be applied at a fairly wet stage (remembering that it will make your work even wetter!). Trying to apply slip to dry clay won’t work! If you want to do sgraffito – you will need to keep the work leather hard (a state of dryness where you can still work the clay with a little effort and a little water and care). Step Three – Drying Most of the time your work can go into the rack uncovered to let it dry out for the following week. If you want to continue forming or shaping you will need to double bag your work – put your work on a suitable sized bat and put the bat in a bag so the base of the bag is under the bat, then put another bag over the top of the work and tuck the top of the bag under the bat. If you want to trim (or turn) your thrown work the following week, it should also be double bagged. If your work is large, delicate, or of uneven thicknesses, you should lightly cover your work for drying. When considering the drying process, bare in mind the weather, humidity and wind! The hotter and dryer, the faster things dry and work can dry unevenly in the shelves – this can lead to cracking – another time to lightly cover your work for drying. Step Four – Trimming and Cleaning Up Your work is dry! It is called greenware now and it is at it’s most fragile! Handle everything with two hands. I often refer to soft hands – keep everything gentle and with your fingers spread as much as possible. Try to not pick up things like plates too much, and always with both hands! Before your work can be bisque fired it should be “cleaned up”. You work won’t go into the kiln if it has sharp edges – when glazed, sharp edges turn into razor blades! Use a piece of fly wire to rub the work all over – this will scratch a little so be light handed. Use a knife or metal kidney to scrape any areas that require a bit more dynamic treatment than the fly wire offers! Finally, a very light wipe over with a slightly damp sponge can help soften and soothe all of your edges and dags! Trimming thrown work: If you are planning to trim (or turn) your thrown work (and you should be), make sure you bag it well – your work should be leather hard to almost dry for easiest trimming. Use this step to finish the work completely – use a metal kidney to polish the surface, or a slightly damp sponge to give a freshly thrown look. Wipe the sponge around the rim after trimming, and check the inside of the pot for dags! Trimming slip cast work: Usually I will trim the rims of your work on the wheel the following day to make that stage easier, however you will still need to check your work for lumps and bumps. Last but not least – check that your name is still clearly on the bottom of your work. Step Five – Bisque Firing When the work is completely dry it can go into the bisque kiln. The bisque kiln is fired to 1000°C. This process burns off the water in the clay as well as some of the chemically bound water. The structure of the clay is not altered that much at this temperature. Inside the bisque kiln, the work is stacked a little, small bowl inside a larger bowl and onto a heavy plate. Smaller items like decorations or drink coasters might get stacked several high. Consideration is paid to the weight of the stack and shape of the work. A bisque kiln can fire about one and a half times the amount of work that the glaze kiln can fire. The firing takes about 10 hours to complete the cycle and about two days to cool down. Once it has been emptied the work is placed in the glaze room ready for you to decorate! Step Six – Glazing Decorating your work with colour can be a lot of fun – and time consuming! There are three main options for surface treatment at this stage: Oxide Washes Underglazes Glazes Washes and underglazes do not “glaze” the work – It will still need a layer of glaze to fully seal the clay (washes don’t need glaze on surfaces not designed for food or liquid as they can gloss up a little on their own). Underglazes are stable colourants that turn out pretty much how they look in the jar. They can be mixed with each other to form other colours and can be used like water colours to paint onto your work. Mostly they should have a clear glaze on top to seal them. Oxides are a different species – the pink oxide (cobalt) wash turns out bright blue for instance. They don’t always need a glaze on top, and some glazes can change the colour of the wash! The glazes need no other “glaze” on top! Be careful of unknown glaze interactions – you can put any combination of glaze in a bowl or on a plate, but only a single glaze on the outside of any vertical surface! Glazes are a chemical reaction under heat. We don’t know the exact chemicals in the Mayco glazes we use. I can guess by the way they interact with each other, however, on the whole, you need to test every idea you have, and not run the test on a vertical surface! Simply put, glaze is a layer of glass like substance that bonds with the clay underneath. Clay is made of silica, alumina and water. Glaze is made of mostly silica. Silica has a melting point of 1700°C and we fire to 1240°C. The silica requires a “flux” to help it melt at the lower temperature. Fluxes can be all sorts of chemicals – a common one is calcium – calcium has a melting point of 2500°C, however, together they both melt at a much lower temperature! Colourants are metal oxides like cobalt (blue), chrome (green through black), copper (green, blue, even red!), manganese (black, purple and pink) iron (red brown), etc. Different chemicals in the glaze can have dramatic effects. for example, barium carbonate (which we don’t use) turns manganese bright pink! Other elements can turn manganese dioxide brown, blue, purple and reddish brown. Manganese dioxide is a flux in and of itself as well. So, glazes that get their black and purple colours, often interact with other glazes and RUN! Our mirror black is a good example – it mixes really well with many glazes because it fluxes them – causes them to melt faster. It will also bring out many beautiful colours in the glazes because it’s black colouring most definitely comes from manganese dioxide! Glaze chemistry is a whole subject on it’s own! We use commercial Mayco glazes on purpose – for their huge range of colour possibilities, stability, cool interactions, artistic freedom with the ability to easily brush the glazes on and ease of use. We currently have almost 50 glazes on hand! A major project is to test the interactions of all glazes with each other. That is 2,500 test tiles!!!! I’m going to make the wall behind the wheels the feature wall of pretty colours! Step Seven – Glaze (Gloss or sometimes called “Glost”) Firing Most of the time this is the final stage of making your creation (but not always!) The glaze kiln goes to 1240°C. This is called cone 6, or midrange. It is the low end of stoneware temperatures. Stoneware clays and glazes are typically fired at cone 8 – 10, that is 1260 – 1290°C. The energy requirement to go from 1240°C to 1280°C is almost a 30% more! Our clay is formulated to vitrify (mature, turn “glass-like”) at 1240°, as are our glazes. A glaze kiln take around 12 hours to reach temperature and two to three days to cool down. Sometimes a third firing process is required – this is for decoration that is added to work after the glaze firing. For example – adding precious metals and lustres. this firing temperature is usually around 600 – 800°C depending upon the techniques being used. There are many students interested in gold and silver trims – we will be doing this third type of firing soon! After firing your work will be in the student finished work shelves. Remember to pay for it before you head out the door! There is a small extra charge for using porcelain clay (it’s more than twice the price of regular clay), and for any third firing process! Once your work has been fired it can not turn back into clay for millennia – so don’t fire it if you don’t like it! Put it in the bucket for recycling. https://firebirdstudios.com.au/the-process-of-making-pottery/ This bowl is an example of kitchenware used in the 19th century and still in use today.Bowl white ceramic plain that has two sets of edging around lip. Inside bowl has plaster designed to look like cooking mixture.flagstaff hill, warrnambool, shipwrecked-coast, flagstaff-hill, flagstaff-hill-maritime-museum, maritime-museum, shipwreck-coast, flagstaff-hill-maritime-village, kitchen equipment, ceramic -
 Flagstaff Hill Maritime Museum and Village
Flagstaff Hill Maritime Museum and VillageDomestic object - Bowl, J & G Meakin, Late 19th or early 20th Century
The Process of Making Pottery Decorating, Firing, Glazing, Making, Technical There is a rhythm and flow to clay. It can’t be done all at once! Even the making process! It can take weeks to get everything done, especially if you can only work on your pottery once a week! Even though we have three hour classes, it’s often just not enough time! Here is an overview of some of the processes so you have a bit more grasp on some of the technical stuff! Step One – Design There are SO many ideas out there for making stuff in clay! From delicate porcelain jewellery, through to heavy sculptural work and everything in between. Deciding your direction is sometimes not that easy – when you first start, try everything, you will naturally gravitate to the style that you enjoy! The options and variations are endless and can get a wee bit overwhelming too! Check in with me before you start to ensure your ideas will work, what order you might do things, how you could achieve the look you are seeking and any other technical data required! Step Two – Making Clay is thixotropic. This means that as you work with it, the clay first gets sloppier and wetter, before is begins to dry in the atmosphere. For most things, you simply can’t do all parts of the project at once. An example of work order might look like: Get last weeks work out from the shelves Prepare clay for today’s work – roll your clay, prepare balls for throwing, make the first stage of a pinch pot) Clean up last week’s work and put it on the shelf for bisque firing Check that you have any glazing to do – and do enough of it that you will have time to finish your main project Do the next step of your next project – there might be a further step that can’t be complete immediately, in that case, wrap your work well and put onto the shelves. Letting your work rest for a while can really help keep your work clean and professional looking. Many things require bagging under plastic to keep it ready for work the next week – put your name on the outside of the bag so you can find your work easily. We have stickers and markers. Consider how you want to decorate your work – coloured slip can be applied at a fairly wet stage (remembering that it will make your work even wetter!). Trying to apply slip to dry clay won’t work! If you want to do sgraffito – you will need to keep the work leather hard (a state of dryness where you can still work the clay with a little effort and a little water and care). Step Three – Drying Most of the time your work can go into the rack uncovered to let it dry out for the following week. If you want to continue forming or shaping you will need to double bag your work – put your work on a suitable sized bat and put the bat in a bag so the base of the bag is under the bat, then put another bag over the top of the work and tuck the top of the bag under the bat. If you want to trim (or turn) your thrown work the following week, it should also be double bagged. If your work is large, delicate, or of uneven thicknesses, you should lightly cover your work for drying. When considering the drying process, bare in mind the weather, humidity and wind! The hotter and dryer, the faster things dry and work can dry unevenly in the shelves – this can lead to cracking – another time to lightly cover your work for drying. Step Four – Trimming and Cleaning Up Your work is dry! It is called greenware now and it is at it’s most fragile! Handle everything with two hands. I often refer to soft hands – keep everything gentle and with your fingers spread as much as possible. Try to not pick up things like plates too much, and always with both hands! Before your work can be bisque fired it should be “cleaned up”. You work won’t go into the kiln if it has sharp edges – when glazed, sharp edges turn into razor blades! Use a piece of fly wire to rub the work all over – this will scratch a little so be light handed. Use a knife or metal kidney to scrape any areas that require a bit more dynamic treatment than the fly wire offers! Finally, a very light wipe over with a slightly damp sponge can help soften and soothe all of your edges and dags! Trimming thrown work: If you are planning to trim (or turn) your thrown work (and you should be), make sure you bag it well – your work should be leather hard to almost dry for easiest trimming. Use this step to finish the work completely – use a metal kidney to polish the surface, or a slightly damp sponge to give a freshly thrown look. Wipe the sponge around the rim after trimming, and check the inside of the pot for dags! Trimming slip cast work: Usually I will trim the rims of your work on the wheel the following day to make that stage easier, however you will still need to check your work for lumps and bumps. Last but not least – check that your name is still clearly on the bottom of your work. Step Five – Bisque Firing When the work is completely dry it can go into the bisque kiln. The bisque kiln is fired to 1000°C. This process burns off the water in the clay as well as some of the chemically bound water. The structure of the clay is not altered that much at this temperature. Inside the bisque kiln, the work is stacked a little, small bowl inside a larger bowl and onto a heavy plate. Smaller items like decorations or drink coasters might get stacked several high. Consideration is paid to the weight of the stack and shape of the work. A bisque kiln can fire about one and a half times the amount of work that the glaze kiln can fire. The firing takes about 10 hours to complete the cycle and about two days to cool down. Once it has been emptied the work is placed in the glaze room ready for you to decorate! Step Six – Glazing Decorating your work with colour can be a lot of fun – and time consuming! There are three main options for surface treatment at this stage: Oxide Washes Underglazes Glazes Washes and underglazes do not “glaze” the work – It will still need a layer of glaze to fully seal the clay (washes don’t need glaze on surfaces not designed for food or liquid as they can gloss up a little on their own). Underglazes are stable colourants that turn out pretty much how they look in the jar. They can be mixed with each other to form other colours and can be used like water colours to paint onto your work. Mostly they should have a clear glaze on top to seal them. Oxides are a different species – the pink oxide (cobalt) wash turns out bright blue for instance. They don’t always need a glaze on top, and some glazes can change the colour of the wash! The glazes need no other “glaze” on top! Be careful of unknown glaze interactions – you can put any combination of glaze in a bowl or on a plate, but only a single glaze on the outside of any vertical surface! Glazes are a chemical reaction under heat. We don’t know the exact chemicals in the Mayco glazes we use. I can guess by the way they interact with each other, however, on the whole, you need to test every idea you have, and not run the test on a vertical surface! Simply put, glaze is a layer of glass like substance that bonds with the clay underneath. Clay is made of silica, alumina and water. Glaze is made of mostly silica. Silica has a melting point of 1700°C and we fire to 1240°C. The silica requires a “flux” to help it melt at the lower temperature. Fluxes can be all sorts of chemicals – a common one is calcium – calcium has a melting point of 2500°C, however, together they both melt at a much lower temperature! Colourants are metal oxides like cobalt (blue), chrome (green through black), copper (green, blue, even red!), manganese (black, purple and pink) iron (red brown), etc. Different chemicals in the glaze can have dramatic effects. for example, barium carbonate (which we don’t use) turns manganese bright pink! Other elements can turn manganese dioxide brown, blue, purple and reddish brown. Manganese dioxide is a flux in and of itself as well. So, glazes that get their black and purple colours, often interact with other glazes and RUN! Our mirror black is a good example – it mixes really well with many glazes because it fluxes them – causes them to melt faster. It will also bring out many beautiful colours in the glazes because it’s black colouring most definitely comes from manganese dioxide! Glaze chemistry is a whole subject on it’s own! We use commercial Mayco glazes on purpose – for their huge range of colour possibilities, stability, cool interactions, artistic freedom with the ability to easily brush the glazes on and ease of use. We currently have almost 50 glazes on hand! A major project is to test the interactions of all glazes with each other. That is 2,500 test tiles!!!! I’m going to make the wall behind the wheels the feature wall of pretty colours! Step Seven – Glaze (Gloss or sometimes called “Glost”) Firing Most of the time this is the final stage of making your creation (but not always!) The glaze kiln goes to 1240°C. This is called cone 6, or midrange. It is the low end of stoneware temperatures. Stoneware clays and glazes are typically fired at cone 8 – 10, that is 1260 – 1290°C. The energy requirement to go from 1240°C to 1280°C is almost a 30% more! Our clay is formulated to vitrify (mature, turn “glass-like”) at 1240°, as are our glazes. A glaze kiln take around 12 hours to reach temperature and two to three days to cool down. Sometimes a third firing process is required – this is for decoration that is added to work after the glaze firing. For example – adding precious metals and lustres. this firing temperature is usually around 600 – 800°C depending upon the techniques being used. There are many students interested in gold and silver trims – we will be doing this third type of firing soon! After firing your work will be in the student finished work shelves. Remember to pay for it before you head out the door! There is a small extra charge for using porcelain clay (it’s more than twice the price of regular clay), and for any third firing process! Once your work has been fired it can not turn back into clay for millennia – so don’t fire it if you don’t like it! Put it in the bucket for recycling. https://firebirdstudios.com.au/the-process-of-making-pottery/This bowl was made by renowned pottery company J & G Meakin of England. The firm was established in the mid-1800's. The bowl is an example of kitchenware used in the 19th century and still in use today.Bowl; white ceramic, round and tapering inwards towards base. Made by J and G Meakin England.On base, 'Ironstone China Reg SOL 391413' with symbolflagstaff hill, flagstaff hill maritime museum and village, warrnambool, maritime museum, maritime village, great ocean road, shipwreck coast, mixing bowl, food preparation, j & g meakin, pottery, stoke-on-trent, kitchen equipment, ceramic -
 Flagstaff Hill Maritime Museum and Village
Flagstaff Hill Maritime Museum and VillageDomestic object - Plate
The Process of Making Pottery Decorating, Firing, Glazing, Making, Technical There is a rhythm and flow to clay. It can’t be done all at once! Even the making process! It can take weeks to get everything done, especially if you can only work on your pottery once a week! Even though we have three hour classes, it’s often just not enough time! Here is an overview of some of the processes so you have a bit more grasp on some of the technical stuff! Step One – Design There are SO many ideas out there for making stuff in clay! From delicate porcelain jewellery, through to heavy sculptural work and everything in between. Deciding your direction is sometimes not that easy – when you first start, try everything, you will naturally gravitate to the style that you enjoy! The options and variations are endless and can get a wee bit overwhelming too! Check in with me before you start to ensure your ideas will work, what order you might do things, how you could achieve the look you are seeking and any other technical data required! Step Two – Making Clay is thixotropic. This means that as you work with it, the clay first gets sloppier and wetter, before is begins to dry in the atmosphere. For most things, you simply can’t do all parts of the project at once. An example of work order might look like: Get last weeks work out from the shelves Prepare clay for today’s work – roll your clay, prepare balls for throwing, make the first stage of a pinch pot) Clean up last week’s work and put it on the shelf for bisque firing Check that you have any glazing to do – and do enough of it that you will have time to finish your main project Do the next step of your next project – there might be a further step that can’t be complete immediately, in that case, wrap your work well and put onto the shelves. Letting your work rest for a while can really help keep your work clean and professional looking. Many things require bagging under plastic to keep it ready for work the next week – put your name on the outside of the bag so you can find your work easily. We have stickers and markers. Consider how you want to decorate your work – coloured slip can be applied at a fairly wet stage (remembering that it will make your work even wetter!). Trying to apply slip to dry clay won’t work! If you want to do sgraffito – you will need to keep the work leather hard (a state of dryness where you can still work the clay with a little effort and a little water and care). Step Three – Drying Most of the time your work can go into the rack uncovered to let it dry out for the following week. If you want to continue forming or shaping you will need to double bag your work – put your work on a suitable sized bat and put the bat in a bag so the base of the bag is under the bat, then put another bag over the top of the work and tuck the top of the bag under the bat. If you want to trim (or turn) your thrown work the following week, it should also be double bagged. If your work is large, delicate, or of uneven thicknesses, you should lightly cover your work for drying. When considering the drying process, bare in mind the weather, humidity and wind! The hotter and dryer, the faster things dry and work can dry unevenly in the shelves – this can lead to cracking – another time to lightly cover your work for drying. Step Four – Trimming and Cleaning Up Your work is dry! It is called greenware now and it is at it’s most fragile! Handle everything with two hands. I often refer to soft hands – keep everything gentle and with your fingers spread as much as possible. Try to not pick up things like plates too much, and always with both hands! Before your work can be bisque fired it should be “cleaned up”. You work won’t go into the kiln if it has sharp edges – when glazed, sharp edges turn into razor blades! Use a piece of fly wire to rub the work all over – this will scratch a little so be light handed. Use a knife or metal kidney to scrape any areas that require a bit more dynamic treatment than the fly wire offers! Finally, a very light wipe over with a slightly damp sponge can help soften and soothe all of your edges and dags! Trimming thrown work: If you are planning to trim (or turn) your thrown work (and you should be), make sure you bag it well – your work should be leather hard to almost dry for easiest trimming. Use this step to finish the work completely – use a metal kidney to polish the surface, or a slightly damp sponge to give a freshly thrown look. Wipe the sponge around the rim after trimming, and check the inside of the pot for dags! Trimming slip cast work: Usually I will trim the rims of your work on the wheel the following day to make that stage easier, however you will still need to check your work for lumps and bumps. Last but not least – check that your name is still clearly on the bottom of your work. Step Five – Bisque Firing When the work is completely dry it can go into the bisque kiln. The bisque kiln is fired to 1000°C. This process burns off the water in the clay as well as some of the chemically bound water. The structure of the clay is not altered that much at this temperature. Inside the bisque kiln, the work is stacked a little, small bowl inside a larger bowl and onto a heavy plate. Smaller items like decorations or drink coasters might get stacked several high. Consideration is paid to the weight of the stack and shape of the work. A bisque kiln can fire about one and a half times the amount of work that the glaze kiln can fire. The firing takes about 10 hours to complete the cycle and about two days to cool down. Once it has been emptied the work is placed in the glaze room ready for you to decorate! Step Six – Glazing Decorating your work with colour can be a lot of fun – and time consuming! There are three main options for surface treatment at this stage: Oxide Washes Underglazes Glazes Washes and underglazes do not “glaze” the work – It will still need a layer of glaze to fully seal the clay (washes don’t need glaze on surfaces not designed for food or liquid as they can gloss up a little on their own). Underglazes are stable colourants that turn out pretty much how they look in the jar. They can be mixed with each other to form other colours and can be used like water colours to paint onto your work. Mostly they should have a clear glaze on top to seal them. Oxides are a different species – the pink oxide (cobalt) wash turns out bright blue for instance. They don’t always need a glaze on top, and some glazes can change the colour of the wash! The glazes need no other “glaze” on top! Be careful of unknown glaze interactions – you can put any combination of glaze in a bowl or on a plate, but only a single glaze on the outside of any vertical surface! Glazes are a chemical reaction under heat. We don’t know the exact chemicals in the Mayco glazes we use. I can guess by the way they interact with each other, however, on the whole, you need to test every idea you have, and not run the test on a vertical surface! Simply put, glaze is a layer of glass like substance that bonds with the clay underneath. Clay is made of silica, alumina and water. Glaze is made of mostly silica. Silica has a melting point of 1700°C and we fire to 1240°C. The silica requires a “flux” to help it melt at the lower temperature. Fluxes can be all sorts of chemicals – a common one is calcium – calcium has a melting point of 2500°C, however, together they both melt at a much lower temperature! Colourants are metal oxides like cobalt (blue), chrome (green through black), copper (green, blue, even red!), manganese (black, purple and pink) iron (red brown), etc. Different chemicals in the glaze can have dramatic effects. for example, barium carbonate (which we don’t use) turns manganese bright pink! Other elements can turn manganese dioxide brown, blue, purple and reddish brown. Manganese dioxide is a flux in and of itself as well. So, glazes that get their black and purple colours, often interact with other glazes and RUN! Our mirror black is a good example – it mixes really well with many glazes because it fluxes them – causes them to melt faster. It will also bring out many beautiful colours in the glazes because it’s black colouring most definitely comes from manganese dioxide! Glaze chemistry is a whole subject on it’s own! We use commercial Mayco glazes on purpose – for their huge range of colour possibilities, stability, cool interactions, artistic freedom with the ability to easily brush the glazes on and ease of use. We currently have almost 50 glazes on hand! A major project is to test the interactions of all glazes with each other. That is 2,500 test tiles!!!! I’m going to make the wall behind the wheels the feature wall of pretty colours! Step Seven – Glaze (Gloss or sometimes called “Glost”) Firing Most of the time this is the final stage of making your creation (but not always!) The glaze kiln goes to 1240°C. This is called cone 6, or midrange. It is the low end of stoneware temperatures. Stoneware clays and glazes are typically fired at cone 8 – 10, that is 1260 – 1290°C. The energy requirement to go from 1240°C to 1280°C is almost a 30% more! Our clay is formulated to vitrify (mature, turn “glass-like”) at 1240°, as are our glazes. A glaze kiln take around 12 hours to reach temperature and two to three days to cool down. Sometimes a third firing process is required – this is for decoration that is added to work after the glaze firing. For example – adding precious metals and lustres. this firing temperature is usually around 600 – 800°C depending upon the techniques being used. There are many students interested in gold and silver trims – we will be doing this third type of firing soon! After firing your work will be in the student finished work shelves. Remember to pay for it before you head out the door! There is a small extra charge for using porcelain clay (it’s more than twice the price of regular clay), and for any third firing process! Once your work has been fired it can not turn back into clay for millennia – so don’t fire it if you don’t like it! Put it in the bucket for recycling. https://firebirdstudios.com.au/the-process-of-making-pottery/Ceramics have evolved over thousands of years.White earthenware dinner plate. Crazing evident all over.Backstamped ‘Made in England S LTD’flagstaff hill, warrnambool, shipwrecked-coast, flagstaff-hill, flagstaff-hill-maritime-museum, maritime-museum, shipwreck-coast, flagstaff-hill-maritime-village, ceramics, tableware -
 Flagstaff Hill Maritime Museum and Village
Flagstaff Hill Maritime Museum and VillageDomestic object - Plate, Johnson Bros
The Process of Making Pottery Decorating, Firing, Glazing, Making, Technical There is a rhythm and flow to clay. It can’t be done all at once! Even the making process! It can take weeks to get everything done, especially if you can only work on your pottery once a week! Even though we have three hour classes, it’s often just not enough time! Here is an overview of some of the processes so you have a bit more grasp on some of the technical stuff! Step One – Design There are SO many ideas out there for making stuff in clay! From delicate porcelain jewellery, through to heavy sculptural work and everything in between. Deciding your direction is sometimes not that easy – when you first start, try everything, you will naturally gravitate to the style that you enjoy! The options and variations are endless and can get a wee bit overwhelming too! Check in with me before you start to ensure your ideas will work, what order you might do things, how you could achieve the look you are seeking and any other technical data required! Step Two – Making Clay is thixotropic. This means that as you work with it, the clay first gets sloppier and wetter, before is begins to dry in the atmosphere. For most things, you simply can’t do all parts of the project at once. An example of work order might look like: Get last weeks work out from the shelves Prepare clay for today’s work – roll your clay, prepare balls for throwing, make the first stage of a pinch pot) Clean up last week’s work and put it on the shelf for bisque firing Check that you have any glazing to do – and do enough of it that you will have time to finish your main project Do the next step of your next project – there might be a further step that can’t be complete immediately, in that case, wrap your work well and put onto the shelves. Letting your work rest for a while can really help keep your work clean and professional looking. Many things require bagging under plastic to keep it ready for work the next week – put your name on the outside of the bag so you can find your work easily. We have stickers and markers. Consider how you want to decorate your work – coloured slip can be applied at a fairly wet stage (remembering that it will make your work even wetter!). Trying to apply slip to dry clay won’t work! If you want to do sgraffito – you will need to keep the work leather hard (a state of dryness where you can still work the clay with a little effort and a little water and care). Step Three – Drying Most of the time your work can go into the rack uncovered to let it dry out for the following week. If you want to continue forming or shaping you will need to double bag your work – put your work on a suitable sized bat and put the bat in a bag so the base of the bag is under the bat, then put another bag over the top of the work and tuck the top of the bag under the bat. If you want to trim (or turn) your thrown work the following week, it should also be double bagged. If your work is large, delicate, or of uneven thicknesses, you should lightly cover your work for drying. When considering the drying process, bare in mind the weather, humidity and wind! The hotter and dryer, the faster things dry and work can dry unevenly in the shelves – this can lead to cracking – another time to lightly cover your work for drying. Step Four – Trimming and Cleaning Up Your work is dry! It is called greenware now and it is at it’s most fragile! Handle everything with two hands. I often refer to soft hands – keep everything gentle and with your fingers spread as much as possible. Try to not pick up things like plates too much, and always with both hands! Before your work can be bisque fired it should be “cleaned up”. You work won’t go into the kiln if it has sharp edges – when glazed, sharp edges turn into razor blades! Use a piece of fly wire to rub the work all over – this will scratch a little so be light handed. Use a knife or metal kidney to scrape any areas that require a bit more dynamic treatment than the fly wire offers! Finally, a very light wipe over with a slightly damp sponge can help soften and soothe all of your edges and dags! Trimming thrown work: If you are planning to trim (or turn) your thrown work (and you should be), make sure you bag it well – your work should be leather hard to almost dry for easiest trimming. Use this step to finish the work completely – use a metal kidney to polish the surface, or a slightly damp sponge to give a freshly thrown look. Wipe the sponge around the rim after trimming, and check the inside of the pot for dags! Trimming slip cast work: Usually I will trim the rims of your work on the wheel the following day to make that stage easier, however you will still need to check your work for lumps and bumps. Last but not least – check that your name is still clearly on the bottom of your work. Step Five – Bisque Firing When the work is completely dry it can go into the bisque kiln. The bisque kiln is fired to 1000°C. This process burns off the water in the clay as well as some of the chemically bound water. The structure of the clay is not altered that much at this temperature. Inside the bisque kiln, the work is stacked a little, small bowl inside a larger bowl and onto a heavy plate. Smaller items like decorations or drink coasters might get stacked several high. Consideration is paid to the weight of the stack and shape of the work. A bisque kiln can fire about one and a half times the amount of work that the glaze kiln can fire. The firing takes about 10 hours to complete the cycle and about two days to cool down. Once it has been emptied the work is placed in the glaze room ready for you to decorate! Step Six – Glazing Decorating your work with colour can be a lot of fun – and time consuming! There are three main options for surface treatment at this stage: Oxide Washes Underglazes Glazes Washes and underglazes do not “glaze” the work – It will still need a layer of glaze to fully seal the clay (washes don’t need glaze on surfaces not designed for food or liquid as they can gloss up a little on their own). Underglazes are stable colourants that turn out pretty much how they look in the jar. They can be mixed with each other to form other colours and can be used like water colours to paint onto your work. Mostly they should have a clear glaze on top to seal them. Oxides are a different species – the pink oxide (cobalt) wash turns out bright blue for instance. They don’t always need a glaze on top, and some glazes can change the colour of the wash! The glazes need no other “glaze” on top! Be careful of unknown glaze interactions – you can put any combination of glaze in a bowl or on a plate, but only a single glaze on the outside of any vertical surface! Glazes are a chemical reaction under heat. We don’t know the exact chemicals in the Mayco glazes we use. I can guess by the way they interact with each other, however, on the whole, you need to test every idea you have, and not run the test on a vertical surface! Simply put, glaze is a layer of glass like substance that bonds with the clay underneath. Clay is made of silica, alumina and water. Glaze is made of mostly silica. Silica has a melting point of 1700°C and we fire to 1240°C. The silica requires a “flux” to help it melt at the lower temperature. Fluxes can be all sorts of chemicals – a common one is calcium – calcium has a melting point of 2500°C, however, together they both melt at a much lower temperature! Colourants are metal oxides like cobalt (blue), chrome (green through black), copper (green, blue, even red!), manganese (black, purple and pink) iron (red brown), etc. Different chemicals in the glaze can have dramatic effects. for example, barium carbonate (which we don’t use) turns manganese bright pink! Other elements can turn manganese dioxide brown, blue, purple and reddish brown. Manganese dioxide is a flux in and of itself as well. So, glazes that get their black and purple colours, often interact with other glazes and RUN! Our mirror black is a good example – it mixes really well with many glazes because it fluxes them – causes them to melt faster. It will also bring out many beautiful colours in the glazes because it’s black colouring most definitely comes from manganese dioxide! Glaze chemistry is a whole subject on it’s own! We use commercial Mayco glazes on purpose – for their huge range of colour possibilities, stability, cool interactions, artistic freedom with the ability to easily brush the glazes on and ease of use. We currently have almost 50 glazes on hand! A major project is to test the interactions of all glazes with each other. That is 2,500 test tiles!!!! I’m going to make the wall behind the wheels the feature wall of pretty colours! Step Seven – Glaze (Gloss or sometimes called “Glost”) Firing Most of the time this is the final stage of making your creation (but not always!) The glaze kiln goes to 1240°C. This is called cone 6, or midrange. It is the low end of stoneware temperatures. Stoneware clays and glazes are typically fired at cone 8 – 10, that is 1260 – 1290°C. The energy requirement to go from 1240°C to 1280°C is almost a 30% more! Our clay is formulated to vitrify (mature, turn “glass-like”) at 1240°, as are our glazes. A glaze kiln take around 12 hours to reach temperature and two to three days to cool down. Sometimes a third firing process is required – this is for decoration that is added to work after the glaze firing. For example – adding precious metals and lustres. this firing temperature is usually around 600 – 800°C depending upon the techniques being used. There are many students interested in gold and silver trims – we will be doing this third type of firing soon! After firing your work will be in the student finished work shelves. Remember to pay for it before you head out the door! There is a small extra charge for using porcelain clay (it’s more than twice the price of regular clay), and for any third firing process! Once your work has been fired it can not turn back into clay for millennia – so don’t fire it if you don’t like it! Put it in the bucket for recycling. https://firebirdstudios.com.au/the-process-of-making-pottery/Ceramics have evolved over thousands of years.A white earthenware side plate with a gadroon edge. Has water marks and chips on front.‘Johnson Bros England Reg No 15587’flagstaff hill, warrnambool, shipwrecked-coast, flagstaff-hill, flagstaff-hill-maritime-museum, maritime-museum, shipwreck-coast, flagstaff-hill-maritime-village, johnson bros, ceramics, tableware -
 Flagstaff Hill Maritime Museum and Village
Flagstaff Hill Maritime Museum and VillageDomestic object - Plate, Alfred Meakin
The Process of Making Pottery Decorating, Firing, Glazing, Making, Technical There is a rhythm and flow to clay. It can’t be done all at once! Even the making process! It can take weeks to get everything done, especially if you can only work on your pottery once a week! Even though we have three hour classes, it’s often just not enough time! Here is an overview of some of the processes so you have a bit more grasp on some of the technical stuff! Step One – Design There are SO many ideas out there for making stuff in clay! From delicate porcelain jewellery, through to heavy sculptural work and everything in between. Deciding your direction is sometimes not that easy – when you first start, try everything, you will naturally gravitate to the style that you enjoy! The options and variations are endless and can get a wee bit overwhelming too! Check in with me before you start to ensure your ideas will work, what order you might do things, how you could achieve the look you are seeking and any other technical data required! Step Two – Making Clay is thixotropic. This means that as you work with it, the clay first gets sloppier and wetter, before is begins to dry in the atmosphere. For most things, you simply can’t do all parts of the project at once. An example of work order might look like: Get last weeks work out from the shelves Prepare clay for today’s work – roll your clay, prepare balls for throwing, make the first stage of a pinch pot) Clean up last week’s work and put it on the shelf for bisque firing Check that you have any glazing to do – and do enough of it that you will have time to finish your main project Do the next step of your next project – there might be a further step that can’t be complete immediately, in that case, wrap your work well and put onto the shelves. Letting your work rest for a while can really help keep your work clean and professional looking. Many things require bagging under plastic to keep it ready for work the next week – put your name on the outside of the bag so you can find your work easily. We have stickers and markers. Consider how you want to decorate your work – coloured slip can be applied at a fairly wet stage (remembering that it will make your work even wetter!). Trying to apply slip to dry clay won’t work! If you want to do sgraffito – you will need to keep the work leather hard (a state of dryness where you can still work the clay with a little effort and a little water and care). Step Three – Drying Most of the time your work can go into the rack uncovered to let it dry out for the following week. If you want to continue forming or shaping you will need to double bag your work – put your work on a suitable sized bat and put the bat in a bag so the base of the bag is under the bat, then put another bag over the top of the work and tuck the top of the bag under the bat. If you want to trim (or turn) your thrown work the following week, it should also be double bagged. If your work is large, delicate, or of uneven thicknesses, you should lightly cover your work for drying. When considering the drying process, bare in mind the weather, humidity and wind! The hotter and dryer, the faster things dry and work can dry unevenly in the shelves – this can lead to cracking – another time to lightly cover your work for drying. Step Four – Trimming and Cleaning Up Your work is dry! It is called greenware now and it is at it’s most fragile! Handle everything with two hands. I often refer to soft hands – keep everything gentle and with your fingers spread as much as possible. Try to not pick up things like plates too much, and always with both hands! Before your work can be bisque fired it should be “cleaned up”. You work won’t go into the kiln if it has sharp edges – when glazed, sharp edges turn into razor blades! Use a piece of fly wire to rub the work all over – this will scratch a little so be light handed. Use a knife or metal kidney to scrape any areas that require a bit more dynamic treatment than the fly wire offers! Finally, a very light wipe over with a slightly damp sponge can help soften and soothe all of your edges and dags! Trimming thrown work: If you are planning to trim (or turn) your thrown work (and you should be), make sure you bag it well – your work should be leather hard to almost dry for easiest trimming. Use this step to finish the work completely – use a metal kidney to polish the surface, or a slightly damp sponge to give a freshly thrown look. Wipe the sponge around the rim after trimming, and check the inside of the pot for dags! Trimming slip cast work: Usually I will trim the rims of your work on the wheel the following day to make that stage easier, however you will still need to check your work for lumps and bumps. Last but not least – check that your name is still clearly on the bottom of your work. Step Five – Bisque Firing When the work is completely dry it can go into the bisque kiln. The bisque kiln is fired to 1000°C. This process burns off the water in the clay as well as some of the chemically bound water. The structure of the clay is not altered that much at this temperature. Inside the bisque kiln, the work is stacked a little, small bowl inside a larger bowl and onto a heavy plate. Smaller items like decorations or drink coasters might get stacked several high. Consideration is paid to the weight of the stack and shape of the work. A bisque kiln can fire about one and a half times the amount of work that the glaze kiln can fire. The firing takes about 10 hours to complete the cycle and about two days to cool down. Once it has been emptied the work is placed in the glaze room ready for you to decorate! Step Six – Glazing Decorating your work with colour can be a lot of fun – and time consuming! There are three main options for surface treatment at this stage: Oxide Washes Underglazes Glazes Washes and underglazes do not “glaze” the work – It will still need a layer of glaze to fully seal the clay (washes don’t need glaze on surfaces not designed for food or liquid as they can gloss up a little on their own). Underglazes are stable colourants that turn out pretty much how they look in the jar. They can be mixed with each other to form other colours and can be used like water colours to paint onto your work. Mostly they should have a clear glaze on top to seal them. Oxides are a different species – the pink oxide (cobalt) wash turns out bright blue for instance. They don’t always need a glaze on top, and some glazes can change the colour of the wash! The glazes need no other “glaze” on top! Be careful of unknown glaze interactions – you can put any combination of glaze in a bowl or on a plate, but only a single glaze on the outside of any vertical surface! Glazes are a chemical reaction under heat. We don’t know the exact chemicals in the Mayco glazes we use. I can guess by the way they interact with each other, however, on the whole, you need to test every idea you have, and not run the test on a vertical surface! Simply put, glaze is a layer of glass like substance that bonds with the clay underneath. Clay is made of silica, alumina and water. Glaze is made of mostly silica. Silica has a melting point of 1700°C and we fire to 1240°C. The silica requires a “flux” to help it melt at the lower temperature. Fluxes can be all sorts of chemicals – a common one is calcium – calcium has a melting point of 2500°C, however, together they both melt at a much lower temperature! Colourants are metal oxides like cobalt (blue), chrome (green through black), copper (green, blue, even red!), manganese (black, purple and pink) iron (red brown), etc. Different chemicals in the glaze can have dramatic effects. for example, barium carbonate (which we don’t use) turns manganese bright pink! Other elements can turn manganese dioxide brown, blue, purple and reddish brown. Manganese dioxide is a flux in and of itself as well. So, glazes that get their black and purple colours, often interact with other glazes and RUN! Our mirror black is a good example – it mixes really well with many glazes because it fluxes them – causes them to melt faster. It will also bring out many beautiful colours in the glazes because it’s black colouring most definitely comes from manganese dioxide! Glaze chemistry is a whole subject on it’s own! We use commercial Mayco glazes on purpose – for their huge range of colour possibilities, stability, cool interactions, artistic freedom with the ability to easily brush the glazes on and ease of use. We currently have almost 50 glazes on hand! A major project is to test the interactions of all glazes with each other. That is 2,500 test tiles!!!! I’m going to make the wall behind the wheels the feature wall of pretty colours! Step Seven – Glaze (Gloss or sometimes called “Glost”) Firing Most of the time this is the final stage of making your creation (but not always!) The glaze kiln goes to 1240°C. This is called cone 6, or midrange. It is the low end of stoneware temperatures. Stoneware clays and glazes are typically fired at cone 8 – 10, that is 1260 – 1290°C. The energy requirement to go from 1240°C to 1280°C is almost a 30% more! Our clay is formulated to vitrify (mature, turn “glass-like”) at 1240°, as are our glazes. A glaze kiln take around 12 hours to reach temperature and two to three days to cool down. Sometimes a third firing process is required – this is for decoration that is added to work after the glaze firing. For example – adding precious metals and lustres. this firing temperature is usually around 600 – 800°C depending upon the techniques being used. There are many students interested in gold and silver trims – we will be doing this third type of firing soon! After firing your work will be in the student finished work shelves. Remember to pay for it before you head out the door! There is a small extra charge for using porcelain clay (it’s more than twice the price of regular clay), and for any third firing process! Once your work has been fired it can not turn back into clay for millennia – so don’t fire it if you don’t like it! Put it in the bucket for recycling. https://firebirdstudios.com.au/the-process-of-making-pottery/Ceramics have evolved over thousands of years.Earthenware dessert plate, cream colour. Made by Alfred Meakin, England. Backstamped ‘Alfred Meakin England’. flagstaff hill, warrnambool, shipwrecked-coast, flagstaff-hill, flagstaff-hill-maritime-museum, maritime-museum, shipwreck-coast, flagstaff-hill-maritime-village, alfred meakin, ceramics, earthenware, kitchenware -
 Flagstaff Hill Maritime Museum and Village
Flagstaff Hill Maritime Museum and VillageDomestic object - Jug
The Process of Making Pottery Decorating, Firing, Glazing, Making, Technical There is a rhythm and flow to clay. It can’t be done all at once! Even the making process! It can take weeks to get everything done, especially if you can only work on your pottery once a week! Even though we have three hour classes, it’s often just not enough time! Here is an overview of some of the processes so you have a bit more grasp on some of the technical stuff! Step One – Design There are SO many ideas out there for making stuff in clay! From delicate porcelain jewellery, through to heavy sculptural work and everything in between. Deciding your direction is sometimes not that easy – when you first start, try everything, you will naturally gravitate to the style that you enjoy! The options and variations are endless and can get a wee bit overwhelming too! Check in with me before you start to ensure your ideas will work, what order you might do things, how you could achieve the look you are seeking and any other technical data required! Step Two – Making Clay is thixotropic. This means that as you work with it, the clay first gets sloppier and wetter, before is begins to dry in the atmosphere. For most things, you simply can’t do all parts of the project at once. An example of work order might look like: Get last weeks work out from the shelves Prepare clay for today’s work – roll your clay, prepare balls for throwing, make the first stage of a pinch pot) Clean up last week’s work and put it on the shelf for bisque firing Check that you have any glazing to do – and do enough of it that you will have time to finish your main project Do the next step of your next project – there might be a further step that can’t be complete immediately, in that case, wrap your work well and put onto the shelves. Letting your work rest for a while can really help keep your work clean and professional looking. Many things require bagging under plastic to keep it ready for work the next week – put your name on the outside of the bag so you can find your work easily. We have stickers and markers. Consider how you want to decorate your work – coloured slip can be applied at a fairly wet stage (remembering that it will make your work even wetter!). Trying to apply slip to dry clay won’t work! If you want to do sgraffito – you will need to keep the work leather hard (a state of dryness where you can still work the clay with a little effort and a little water and care). Step Three – Drying Most of the time your work can go into the rack uncovered to let it dry out for the following week. If you want to continue forming or shaping you will need to double bag your work – put your work on a suitable sized bat and put the bat in a bag so the base of the bag is under the bat, then put another bag over the top of the work and tuck the top of the bag under the bat. If you want to trim (or turn) your thrown work the following week, it should also be double bagged. If your work is large, delicate, or of uneven thicknesses, you should lightly cover your work for drying. When considering the drying process, bare in mind the weather, humidity and wind! The hotter and dryer, the faster things dry and work can dry unevenly in the shelves – this can lead to cracking – another time to lightly cover your work for drying. Step Four – Trimming and Cleaning Up Your work is dry! It is called greenware now and it is at it’s most fragile! Handle everything with two hands. I often refer to soft hands – keep everything gentle and with your fingers spread as much as possible. Try to not pick up things like plates too much, and always with both hands! Before your work can be bisque fired it should be “cleaned up”. You work won’t go into the kiln if it has sharp edges – when glazed, sharp edges turn into razor blades! Use a piece of fly wire to rub the work all over – this will scratch a little so be light handed. Use a knife or metal kidney to scrape any areas that require a bit more dynamic treatment than the fly wire offers! Finally, a very light wipe over with a slightly damp sponge can help soften and soothe all of your edges and dags! Trimming thrown work: If you are planning to trim (or turn) your thrown work (and you should be), make sure you bag it well – your work should be leather hard to almost dry for easiest trimming. Use this step to finish the work completely – use a metal kidney to polish the surface, or a slightly damp sponge to give a freshly thrown look. Wipe the sponge around the rim after trimming, and check the inside of the pot for dags! Trimming slip cast work: Usually I will trim the rims of your work on the wheel the following day to make that stage easier, however you will still need to check your work for lumps and bumps. Last but not least – check that your name is still clearly on the bottom of your work. Step Five – Bisque Firing When the work is completely dry it can go into the bisque kiln. The bisque kiln is fired to 1000°C. This process burns off the water in the clay as well as some of the chemically bound water. The structure of the clay is not altered that much at this temperature. Inside the bisque kiln, the work is stacked a little, small bowl inside a larger bowl and onto a heavy plate. Smaller items like decorations or drink coasters might get stacked several high. Consideration is paid to the weight of the stack and shape of the work. A bisque kiln can fire about one and a half times the amount of work that the glaze kiln can fire. The firing takes about 10 hours to complete the cycle and about two days to cool down. Once it has been emptied the work is placed in the glaze room ready for you to decorate! Step Six – Glazing Decorating your work with colour can be a lot of fun – and time consuming! There are three main options for surface treatment at this stage: Oxide Washes Underglazes Glazes Washes and underglazes do not “glaze” the work – It will still need a layer of glaze to fully seal the clay (washes don’t need glaze on surfaces not designed for food or liquid as they can gloss up a little on their own). Underglazes are stable colourants that turn out pretty much how they look in the jar. They can be mixed with each other to form other colours and can be used like water colours to paint onto your work. Mostly they should have a clear glaze on top to seal them. Oxides are a different species – the pink oxide (cobalt) wash turns out bright blue for instance. They don’t always need a glaze on top, and some glazes can change the colour of the wash! The glazes need no other “glaze” on top! Be careful of unknown glaze interactions – you can put any combination of glaze in a bowl or on a plate, but only a single glaze on the outside of any vertical surface! Glazes are a chemical reaction under heat. We don’t know the exact chemicals in the Mayco glazes we use. I can guess by the way they interact with each other, however, on the whole, you need to test every idea you have, and not run the test on a vertical surface! Simply put, glaze is a layer of glass like substance that bonds with the clay underneath. Clay is made of silica, alumina and water. Glaze is made of mostly silica. Silica has a melting point of 1700°C and we fire to 1240°C. The silica requires a “flux” to help it melt at the lower temperature. Fluxes can be all sorts of chemicals – a common one is calcium – calcium has a melting point of 2500°C, however, together they both melt at a much lower temperature! Colourants are metal oxides like cobalt (blue), chrome (green through black), copper (green, blue, even red!), manganese (black, purple and pink) iron (red brown), etc. Different chemicals in the glaze can have dramatic effects. for example, barium carbonate (which we don’t use) turns manganese bright pink! Other elements can turn manganese dioxide brown, blue, purple and reddish brown. Manganese dioxide is a flux in and of itself as well. So, glazes that get their black and purple colours, often interact with other glazes and RUN! Our mirror black is a good example – it mixes really well with many glazes because it fluxes them – causes them to melt faster. It will also bring out many beautiful colours in the glazes because it’s black colouring most definitely comes from manganese dioxide! Glaze chemistry is a whole subject on it’s own! We use commercial Mayco glazes on purpose – for their huge range of colour possibilities, stability, cool interactions, artistic freedom with the ability to easily brush the glazes on and ease of use. We currently have almost 50 glazes on hand! A major project is to test the interactions of all glazes with each other. That is 2,500 test tiles!!!! I’m going to make the wall behind the wheels the feature wall of pretty colours! Step Seven – Glaze (Gloss or sometimes called “Glost”) Firing Most of the time this is the final stage of making your creation (but not always!) The glaze kiln goes to 1240°C. This is called cone 6, or midrange. It is the low end of stoneware temperatures. Stoneware clays and glazes are typically fired at cone 8 – 10, that is 1260 – 1290°C. The energy requirement to go from 1240°C to 1280°C is almost a 30% more! Our clay is formulated to vitrify (mature, turn “glass-like”) at 1240°, as are our glazes. A glaze kiln take around 12 hours to reach temperature and two to three days to cool down. Sometimes a third firing process is required – this is for decoration that is added to work after the glaze firing. For example – adding precious metals and lustres. this firing temperature is usually around 600 – 800°C depending upon the techniques being used. There are many students interested in gold and silver trims – we will be doing this third type of firing soon! After firing your work will be in the student finished work shelves. Remember to pay for it before you head out the door! There is a small extra charge for using porcelain clay (it’s more than twice the price of regular clay), and for any third firing process! Once your work has been fired it can not turn back into clay for millennia – so don’t fire it if you don’t like it! Put it in the bucket for recycling. https://firebirdstudios.com.au/the-process-of-making-pottery/The form of the jug has been in use for many centuries.Stoneware jug. Two tone brown glaze with pierced lip behind spout. Spout chipped.None.flagstaff hill, warrnambool, shipwrecked-coast, flagstaff-hill, flagstaff-hill-maritime-museum, maritime-museum, shipwreck-coast, flagstaff-hill-maritime-village, jug, ceramic jug -
 Orbost & District Historical Society
Orbost & District Historical Societytobacco cutter, c. mid 19th century
Used to cut tobacco leaf or plugs into finer and more useable samples which could then be put into a pipe or made into a cigar or cigarette.Tobacco cutters were important tools for pipe smokers until self-made or manufactured cigarettes began to dominate the tobacco sales market from the 1920s. This item is a link to a previously common means of consuming tobacco.A metal blade cutter set onto a wooden base. The blade is rusty. tobacco-cutter tobacco-smoking
