Showing 462 items matching " liquid"
-
 Flagstaff Hill Maritime Museum and Village
Flagstaff Hill Maritime Museum and VillageContainer - Bottle, 1840s to 1910
This handmade ‘gallon’ style of bottle was generally used for storing and transporting wine and ale. Many bottles similar to this one have their bases embossed with “6 TO THE GALLON”. It is one of many artefacts recovered from unidentified shipwrecks along Victoria’s coast between the late 1960s and the early 1970s. It is now part of the John Chance Collection. The capacity of this is one-sixth of a gallon (imperial measure), which is equal to 758 ml. (American bottles were often inscribed “5 TO THE GALLON”, which is one-fifth of an American gallon, equal to 757 ml.) Contemporary home brewers can purchase new ‘6 to gallon’ bottles that hold 750 ml. and are sold in cases of 36 bottles, which is equal to 6 gallons of wine. Glass was made thousands of years ago by heating together quartz-sand (Silica), lime and potash. Potash was obtained from burnt wood, but these days potash is mined. The natural sand had imperfections such as different forms of iron, resulting in ‘black’ glass, which was really dark green or dark amber colour. The ‘black’ glass was enhanced by residual carbon in the potash. Black glass is rarely used nowadays but most beer, wine, and liquors are still sold in dark coloured glass. Glass vessels were core-formed from around 1500 BC. An inner core with the vessel’s shape was formed around a rod using a porous material such as clay or dung. Molten glass was then modelled around the core and decorated. When the glass had cooled the vessel was immersed in water and the inner core became liquid and was washed out. Much more recently, bottlers were crafted by a glassblower using molten glass and a blow pipe together with other hand tools. Another method was using simple moulds, called dip moulds, that allowed the glass to be blown into the mould to form the base, then the glassblower would continue blowing free-form to shape the shoulders and neck. The bottle was then finished by applying a lip. These moulded bottles were more uniform in shape compared to the free-form bottles originally produced. English glassblowers in the mid-1800s were making some bottles with 2-piece and 3-piece moulds, some with a push-up style base, sometimes with embossing in the base as well. Improvements allowed the moulds to also have embossed and patterned sides, and straight sided shapes such as hexagons. Bottles made in full moulds usually displayed seam seams or lines. These process took skill and time, making the bottles valuable, so they were often recycled. By the early 20th century bottles were increasingly machine made, which greatly reduced the production time and cost. This bottle is historically significant as an example of a handmade, blown inscribed glass bottle manufactured in the mid-to-late 1800s for specific use as a liquor bottle with a set measurement of one-sixth of gallon. It is also historically significant as an example of liquor bottles imported into Colonial Victoria in the mid-to-late 1800s, giving a snapshot into history and social life that occurred during the early days of Victoria’s development, and the sea trade that visited the ports in those days. The bottle is also significant as one of a group of bottles recovered by John Chance, a diver in Victoria’s coastal waters in the late 1960s to early 1970s. Items that come from several wrecks have since been donated to the Flagstaff Hill Maritime Village’s museum collection of shipwreck artefacts by his family, illustrating this item’s level of historical value. Bottle, olive green glass, handmade. Tall slim Gallon style liquor bottle. Applied double collar lip; square upper and flared lower. Mouth has remnants of tape and wire seal. Mould seam around shoulder. Body tapers slightly inward to the base. Push-up base has pontil mark and is embossed in large letters. Base is uneven. Embossed on base "6 TO THE GALLON"flagstaff hill, warrnambool, flagstaff hill maritime museum, maritime museum, shipwreck coast, flagstaff hill maritime village, great ocean road, shipwreck artefact, john chance, glass bottle, antique bottle, gallon bottle, 6 to the gallon bottle, handmade, dip mould, mouth blown, pontil mark, blown bottle, liquor bottle, ale bottle, double collar, 19th century bottle, collectable -
 Flagstaff Hill Maritime Museum and Village
Flagstaff Hill Maritime Museum and VillageContainer - Bottle, 1840s to 1910
This handmade ‘gallon’ style of bottle was generally used for storing and transporting wine and ale. Many bottles similar to this one have their bases embossed with “6 TO THE GALLON”. It is one of many artefacts recovered from an unidentified shipwrecks along Victoria’s coast between the late 1960s and the early 1970s. It is now part of the John Chance Collection. The capacity of this is one-sixth of a gallon (imperial measure), which is equal to 758 ml. (American bottles were often inscribed “5 TO THE GALLON”, which is one-fifth of an American gallon, equal to 757 ml.) Contemporary home brewers can purchase new ‘6 to gallon’ bottles that hold 750 ml. and are sold in cases of 36 bottles, which is equal to 6 gallons of wine. Glass was made thousands of years ago by heating together quartz-sand (Silica), lime and potash. Potash was obtained from burnt wood, but these days potash is mined. The natural sand had imperfections such as different forms of iron, resulting in ‘black’ glass, which was really dark green or dark amber colour. The ‘black’ glass was enhanced by residual carbon in the potash. Black glass is rarely used nowadays but most beer, wine, and liquors are still sold in dark coloured glass. Glass vessels were core-formed from around 1500 BC. An inner core with the vessel’s shape was formed around a rod using a porous material such as clay or dung. Molten glass was then modelled around the core and decorated. When the glass had cooled the vessel was immersed in water and the inner core became liquid and was washed out. Much more recently, bottlers were crafted by a glassblower using molten glass and a blow pipe together with other hand tools. Another method was using simple moulds, called dip moulds, that allowed the glass to be blown into the mould to form the base, then the glassblower would continue blowing free-form to shape the shoulders and neck. The bottle was then finished by applying a lip. These moulded bottles were more uniform in shape compared to the free-form bottles originally produced. English glassblowers in the mid-1800s were making some bottles with 2-piece and 3-piece moulds, some with a push-up style base, sometimes with embossing in the base as well. Improvements allowed the moulds to also have embossed and patterned sides, and straight sided shapes such as hexagons. Bottles made in full moulds usually displayed seam seams or lines. These process took skill and time, making the bottles valuable, so they were often recycled. By the early 20th century bottles were increasingly machine made, which greatly reduced the production time and cost. This bottle is historically significant as an example of a handmade, blown inscribed glass bottle manufactured in the mid-to-late 1800s for specific use as a liquor bottle with a set measurement of one-sixth of gallon. It is also historically significant as an example of liquor bottles imported into Colonial Victoria in the mid-to-late 1800s, giving a snapshot into history and social life that occurred during the early days of Victoria’s development, and the sea trade that visited the ports in those days. The bottle is also significant as one of a group of bottles recovered by John Chance, a diver in Victoria’s coastal waters in the late 1960s to early 1970s. Items that come from several wrecks have since been donated to the Flagstaff Hill Maritime Village’s museum collection of shipwreck artefacts by his family, illustrating this item’s level of historical value. Bottle, brown glass, Tall slim gallon style. Applied double collar lip; upper is straight, lower is flared. Lip has bumps around the top. Neck has slight taper towards shoulder, which has a shoulder seam from the mould. Body tapers inwards towards base. Push up base has a pontil mark. Base is embossed.Embossed on base "6 TO THE GALLON"flagstaff hill, warrnambool, flagstaff hill maritime museum, maritime museum, shipwreck coast, flagstaff hill maritime village, great ocean road, shipwreck artefact, john chance, glass bottle, antique bottle, gallon bottle, 6 to the gallon bottle, handmade, dip mould, mouth blown, pontil mark, blown bottle, liquor bottle, ale bottle, double collar, 19th century bottle, collectable -
 Flagstaff Hill Maritime Museum and Village
Flagstaff Hill Maritime Museum and VillageContainer - Bottle, 1840s to 1910
This handmade ‘gallon’ style of bottle was generally used for storing and transporting wine and ale. Many bottles similar to this one have their bases embossed with “6 TO THE GALLON”. However, this bottle is rare, in that the base has been embossed then over-embossed with the same text, letters overlapping. It is one of many artefacts recovered from unidentified shipwrecks along Victoria’s coast between the late 1960s and the early 1970s. It is now part of the John Chance Collection. The capacity of this is one-sixth of a gallon (imperial measure), which is equal to 758 ml. (American bottles were often inscribed “5 TO THE GALLON”, which is one-fifth of an American gallon, equal to 757 ml.) Contemporary home brewers can purchase new ‘6 to gallon’ bottles that hold 750 ml. and are sold in cases of 36 bottles, which is equal to 6 gallons of wine. Glass was made thousands of years ago by heating together quartz-sand (Silica), lime and potash. Potash was obtained from burnt wood, but these days potash is mined. The natural sand had imperfections such as different forms of iron, resulting in ‘black’ glass, which was really dark green or dark amber colour. The ‘black’ glass was enhanced by residual carbon in the potash. Black glass is rarely used nowadays but most beer, wine, and liquors are still sold in dark coloured glass. Glass vessels were core-formed from around 1500 BC. An inner core with the vessel’s shape was formed around a rod using a porous material such as clay or dung. Molten glass was then modelled around the core and decorated. When the glass had cooled the vessel was immersed in water and the inner core became liquid and was washed out. Much more recently, bottlers were crafted by a glassblower using molten glass and a blow pipe together with other hand tools. Another method was using simple moulds, called dip moulds, that allowed the glass to be blown into the mould to form the base, then the glassblower would continue blowing free-form to shape the shoulders and neck. The bottle was then finished by applying a lip. These moulded bottles were more uniform in shape compared to the free-form bottles originally produced. English glassblowers in the mid-1800s were making some bottles with 2-piece and 3-piece moulds, some with a push-up style base, sometimes with embossing in the base as well. Improvements allowed the moulds to also have embossed and patterned sides, and straight sided shapes such as hexagons. Bottles made in full moulds usually displayed seam seams or lines. These process took skill and time, making the bottles valuable, so they were often recycled. By the early 20th century bottles were increasingly machine made, which greatly reduced the production time and cost. This bottle is a rare find, in that the base has been over-embossed with the same lettering, letters overlapping one another. This bottle is historically significant as an example of a handmade, blown inscribed glass bottle manufactured in the mid-to-late 1800s for specific use as a liquor bottle with a set measurement of one-sixth of gallon. It is also historically significant as an example of liquor bottles imported into Colonial Victoria in the mid-to-late 1800s, giving a snapshot into history and social life that occurred during the early days of Victoria’s development, and the sea trade that visited the ports in those days. The bottle is also significant as one of a group of bottles recovered by John Chance, a diver in Victoria’s coastal waters in the late 1960s to early 1970s. Items that come from several wrecks have since been donated to the Flagstaff Hill Maritime Village’s museum collection of shipwreck artefacts by his family, illustrating this item’s level of historical value. Bottle, over embossed, brown glass, handmade, rare. Tall slim Gallon style liquor bottle. Applied double collar lip; square upper and flared lower. Mouth has sealing tape remnants around top. Mould seam around shoulder. Body tapers inwards to push-up base. Top edge of lip has application faults. There is also a rectangular indent in the upper edge of lip. Base is embossed and over embossed, with the letters overlapping each other. Embossed on base "6 TO THE GALLON", then over-embossed with the same "6 TO THE GALLON"flagstaff hill, warrnambool, flagstaff hill maritime museum, maritime museum, shipwreck coast, flagstaff hill maritime village, great ocean road, shipwreck artefact, john chance, glass bottle, antique bottle, gallon bottle, 6 to the gallon bottle, handmade, dip mould, mouth blown, pontil mark, blown bottle, liquor bottle, ale bottle, double collar, 19th century bottle, collectable, over embossed, rare -
 Flagstaff Hill Maritime Museum and Village
Flagstaff Hill Maritime Museum and VillageAccessory - Gold Cross, Bef. 01-06-1878
The gold cross was discovered by Victorian scuba diver Julie Wilkins, who had already experienced more than 500 dives in Australia and overseas. She was holidaying in Peterborough, Victoria, and looking forward to discovering more about the famous Loch Ard ship, wrecked in June 1878 at Mutton Bird Island. The fast Glasgow-built clipper ship was only five years old when the tragedy occurred. There were 54 people on board the vessel and only two survived Julie's holiday photograph of Boat Bay reminds her of her most memorable dive. Submerged in the calm, flat sea, she was carefully scanning around the remains of the old wreck when, to her amazement, a gold coin and a small gold cross suddenly came up towards her. She excitedly cupped them in her hands, then stowed the treasures safely in her wetsuit and continued her dive. She soon discovered a group of brass carriage clock parts and some bottles of champagne. It was a day full of surprises. The items were easily recognisable, without any build-up of encrustations or concretion. Julie secretly enjoyed her treasures for twenty-four years then packed them up for the early morning train trip to Warrnambool. After a short walk to Flagstaff Hill Maritime Museum and Village, her photograph was taken as she handed over her precious find. She told her story to a local newspaper reporter, lunched a café in town then took the late afternoon train home. Her generous donation is now part of a vast collection of Loch Ard shipwreck artefacts, including the gold watch and the Minton Majolica model peacock. The small decorative cross dates back to on or before 1878, when the Loch Ard had set sail. The loop and ring have been added, perhaps as a pendant, pocket watch accessory or similar purpose. It may have been worn for ‘good luck’ or a ‘blessing’ on the long journey to Australia, where ships had to carefully navigate the treacherous Bass’s Strait before arriving at their destination of Melbourne. Sadly, many met their fate on that short stretch of ocean aptly named the Shipwreck Coast. The cross is very recognisable even though it was exposed to the wrecking of the ship, its consequent movement, and the sea's turbulence. Its scratched, pitted and worn condition, and the damage near the loop, is part of its story. The red-brown-black discolouration is similar to that found on other gold coins, sometimes called the ‘corrosion phenomena’. Studies suggest the possible cause is contaminants in the minting process reacting to the coins’ environment. Three edges of the cross have slightly raised narrow ridges of gold which could have been cause by the gold being cast liquid gold into a mould.This gold cross pendant is significant as a symbol of Christianity, a sign of hope and safety, and a sample of the religious following on board the Loch Ard, although not everyone wears a cross for this reason. This cross is a sample of jewellery owned by people migrating to Australia in the late 19th century. The cross and the guinea recovered together from the wreck of the Loch Ard are made of gold and help interpret the financial status of some of those on board.Gold cross; yellow gold with decorative hand engraved foliage design on the front, fitted loop and ring on top. The simple Latin or Roman variation of the cross, with an elongated vertical arm, has no figure on it and the reverse has no decoration. The right, left and base edges have sections of narrow, long slightly raised ridges. The top edge has remnants of red-black colour. Victorian era cross, ca. 1878. The cross was recovered from the wreck of the ship Loch Ard.Engraved foliage design. Slightly raised long ridges on sides and base edges. flagstaff hill maritime museum and village, warrnambool, great ocean road, shipwreck coast, gold cross, religious cross, religious trinket, religious jewellery, engraved cross, cross pendant, cross with ring, victorian era, 1878, antique cross, crucifix, religious symbol, christian symbol, christian jewellery, contamination phenomena, gold corrosion, good luck, lucky charm, blessing, pendant, loch ard, wreck of the loch ard, mutton bird island, peterborough, scuba diver, 1980s, shipwreck artefact, relic, latin cross, roman cross, pectoral cross, julie wilkins -
 Flagstaff Hill Maritime Museum and Village
Flagstaff Hill Maritime Museum and VillageContainer - Ship Tank, Early 20th century
Cubed iron ship tanks were invented by Richard Trevithich in 1808, in partnership with Robert Dickson, and the design was patented that year. The invention eventually replaced the wooden casks or barrels used at the time, as the ship tanks were more secure and their shape was much more economical in storage space. The robust metal tanks were originally made to transport water, but their preserving quality enabled them to transport perishable dry goods such as grain on long voyages, as well as other forms of liquid and solid cargo. The first ship tanks were made from sheets of iron, and later mild steel sheets, with double riveted edges and corners. A round cast iron lid with handle grips was fitted snugly into the top opening, providing an air-tight and water-tight seal. Brass taps were often fitted into the base, which could have been the case with this tank, but it now has a wooden bung in the hole. Sailing ships began using the new ship tanks on Australian voyages from around the 1830s, storing food and water for those on board, and filling them with other cargo. A large number of the ship tanks were repurposed in 1838 for the Victoria Settlement at Port Essington, N.T., as they were able to protect the food, clothing and other stores from termite and insect damage. Other ‘recycled’ ship tanks were cut in half and used as washing tubs or cookers. A 1929 catalogue from Hudson’s Tank Stores advertised square tanks that contained around 600 litres – 160 gallons – which equals an internal measurement of 33.25 inches – 84.5 centimetres – per side ship tanks are still made and sold in 1952 by the Globe Tank and Foundry in Wolverhampton, England, which was incorporated in 1922. Today, ship tanks can be seen around Australia. Wilsonson’s Promontory Lightstation has the lid from a ship tank that was used on site for the storage of water. Some have been repurposed as domestic water tanks and dog kennels, others for eucalyptus distilleries. Flagstaff Hill has two ship tanks. The Campaspe Port at Echuca, once a bustling river port, has a ship tank beside the locomotive yard. The lids of ship tanks are collectible items. Richard Trevithick (1771-1833): - Trevithick was born in 1771 in Cornwall, England. He was a famous British engineer and inventor during the Industrial Revolution, known for his invention of the first full-scale working railway, the high-pressure steam locomotive, which he demonstrated hauling a railway train in 1804. He set up a small workshop in 1808 at 72 Fore Street, Limehouse, London, to make iron ship tanks, and this invention was instrumental in replacing the wooden casks formerly used for storage on ships. He was involved with mining technology, iron foundry and ship equipment. Ship tanks changed the way that cargo was transported on ships and other vehicles from the 1830s and were used into the mid-20th century. The ship tanks’ advantages were that they could store more content, lasted longer, were waterproof and airtight, stackable and could be repurposed for many uses other than water, such as fuel, dry goods and domestic cargo. They have even been used aa dog kennels and cookers. Ship tanks were part of the evolving methos to transport water, food and cargo, which changed in 1956 when Malcolm McLean invented the large, rectangular shipping containers that are in use today; they speed up the process of loading on and off the ships, saving time and money. Ship tank: a cubed iron container with an offset hole on one side. The hole has cutouts for securely attaching a lid. This tank is on its side on the ground with the opening facing sideways. The side facing upwards has a wooden bung in a round hole. The ship tank is made from six square, thick iron sheets, rolled and riveted along the edges. The inside has a black sticky coating, possibly bitumen, and a strong creosote odour. There are small remnants of green paint on the outer surface.flagstaff hill, flagstaff hill maritime museum and village, warrnambool, maritime museum, maritime village, great ocean road, shipwreck coast, iron cube, water tank, shipping container, ship tank, ship's tank, ship tanks, marine container, richard trevithich, 1808, robert dickson, water transport, water storage, iron foundry, steel sheets, iron sheets, revets, victoria settlement, port essington, globe tank and foundry, wolverhampton, british engineer, british inventor, wooden casks -
 Flagstaff Hill Maritime Museum and Village
Flagstaff Hill Maritime Museum and VillageFunctional object - Glass Measuring Tube
A graduated cylinder, also known as a measuring cylinder or mixing cylinder, is a common piece of laboratory equipment used to measure the volume of a liquid. It has a narrow cylindrical shape. Each marked line on the graduated cylinder represents the amount of liquid that has been measured. A traditional graduated cylinder is usually narrow and tall so as to increase the accuracy and precision of volume measurement. It has a plastic or glass base (stand, foot, support) and a "spout" for easy pouring of the measured liquid. https://en.wikipedia.org/wiki/Graduated_cylinder The glass measuring tube was donated to Flagstaff Hill Maritime Village by the family of Doctor William Roy Angus, Surgeon and Oculist. It is part of the “W.R. Angus Collection” includes historical medical equipment, surgical instruments and material once belonging to Dr Edward Ryan and Dr Thomas Francis Ryan, (both of Nhill, Victoria) as well as Dr Angus’ own belongings. The Collection’s history spans the medical practices of the two Doctors Ryan, from 1885-1926 plus that of Dr Angus, up until 1969. ABOUT THE “W.R.ANGUS COLLECTION” Doctor William Roy Angus M.B., B.S., Adel., 1923, F.R.C.S. Edin.,1928 (also known as Dr Roy Angus) was born in Murrumbeena, Victoria in 1901 and lived until 1970. He qualified as a doctor in 1923 at University of Adelaide, was Resident Medical Officer at the Royal Adelaide Hospital in 1924 and for a period was house surgeon to Sir (then Mr.) Henry Simpson Newland. Dr Angus was briefly an Assistant to Dr Riddell of Kapunda, then commenced private practice at Curramulka, Yorke Peninsula, SA, where he was physician, surgeon and chemist. In 1926, he was appointed as new Medical Assistant to Dr Thomas Francis Ryan (T.F. Ryan, or Tom), in Nhill, Victoria, where his experiences included radiology and pharmacy. In 1927 he was Acting House Surgeon in Dr Tom Ryan’s absence. Dr Angus had become engaged to Gladys Forsyth and they decided he further his studies overseas in the UK in 1927. He studied at London University College Hospital and at Edinburgh Royal Infirmary and in 1928, was awarded FRCS (Fellow from the Royal College of Surgeons), Edinburgh. He worked his passage back to Australia as a Ship’s Surgeon on the on the Australian Commonwealth Line’s T.S.S. Largs Bay. Dr Angus married Gladys in 1929, in Ballarat. (They went on to have one son (Graham 1932, born in SA) and two daughters (Helen (died 12/07/1996) and Berenice (Berry), both born at Mira, Nhill ) According to Berry, her mother Gladys made a lot of their clothes. She was very talented and did some lovely embroidery including lingerie for her trousseau and beautifully handmade baby clothes. Dr Angus was a ‘flying doctor’ for the A.I.M. (Australian Inland Ministry) Aerial Medical Service in 1928 . Its first station was in the remote town of Oodnadatta, where Dr Angus was stationed. He was locum tenens there on North-South Railway at 21 Mile Camp. He took up this ‘flying doctor’ position in response to a call from Dr John Flynn; the organisation was later known as the Flying Doctor Service, then the Royal Flying Doctor Service. A lot of his work during this time involved dental surgery also. Between 1928-1932 he was surgeon at the Curramulka Hospital, Yorke Peninsula, South Australia. In 1933 Dr Angus returned to Nhill and purchased a share of the Nelson Street practice and Mira hospital (a 2 bed ward at the Nelson Street Practice) from Dr Les Middleton one of the Middleton Brothers, the current owners of what previously once Dr Tom Ryan’s practice. Dr Tom and his brother had worked as surgeons included eye surgery. Dr Tom Ryan performed many of his operations in the Mira private hospital on his premises. He had been House Surgeon at the Nhill Hospital 1902-1926. Dr Tom Ryan had one of the only two pieces of radiology equipment in Victoria during his practicing years – The Royal Melbourne Hospital had the other one. Over the years Dr Tom Ryan had gradually set up what was effectively a training school for country general-practitioner-surgeons. Each patient was carefully examined, including using the X-ray machine, and any surgery was discussed and planned with Dr Ryan’s assistants several days in advance. Dr Angus gained experience in using the X-ray machine there during his time as assistant to Dr Ryan. When Dr Angus bought into the Nelson Street premises in Nhill he was also appointed as the Nhill Hospital’s Honorary House Surgeon 1933-1938. His practitioner’s plate from his Nhill surgery is now mounted on the doorway to the Port Medical Office at Flagstaff Hill Maritime Village, Warrnambool. When Dr Angus took up practice in the Dr Edward and Dr Tom Ryan’s old premises he obtained their extensive collection of historical medical equipment and materials spanning 1884-1926. A large part of this collection is now on display at the Port Medical Office at Flagstaff Hill Maritime Village in Warrnambool. In 1939 Dr Angus and his family moved to Warrnambool where he purchased “Birchwood,” the 1852 home and medical practice of Dr John Hunter Henderson, at 214 Koroit Street. (This property was sold in1965 to the State Government and is now the site of the Warrnambool Police Station. and an ALDI sore is on the land that was once their tennis court). The Angus family was able to afford gardeners, cooks and maids; their home was a popular place for visiting dignitaries to stay whilst visiting Warrnambool. Dr Angus had his own silk worm farm at home in a Mulberry tree. His young daughter used his centrifuge for spinning the silk. Dr Angus was appointed on a part-time basis as Port Medical Officer (Health Officer) in Warrnambool and held this position until the 1940’s when the government no longer required the service of a Port Medical Officer in Warrnambool; he was thus Warrnambool’s last serving Port Medical Officer. (Masters of immigrant ships arriving in port reported incidents of diseases, illness and death and the Port Medical Officer made a decision on whether the ship required Quarantine and for how long, in this way preventing contagious illness from spreading from new immigrants to the residents already in the colony.) Dr Angus was a member of the Australian Medical Association, for 35 years and surgeon at the Warrnambool Base Hospital 1939-1942, He served with the Australian Department of Defence as a Surgeon Captain during WWII 1942-45, in Ballarat, Victoria, and in Bonegilla, N.S.W., completing his service just before the end of the war due to suffering from a heart attack. During his convalescence he carved an intricate and ‘most artistic’ chess set from the material that dentures were made from. He then studied ophthalmology at the Royal Melbourne Eye and Ear Hospital and created cosmetically superior artificial eyes by pioneering using the intrascleral cartilage. Angus received accolades from the Ophthalmological Society of Australasia for this work. He returned to Warrnambool to commence practice as an ophthalmologist, pioneering in artificial eye improvements. He was Honorary Consultant Ophthalmologist to Warrnambool Base Hospital for 31 years. He made monthly visits to Portland as a visiting surgeon, to perform eye surgery. He represented the Victorian South-West subdivision of the Australian Medical Association as its secretary between 1949 and 1956 and as chairman from 1956 to 1958. In 1968 Dr Angus was elected member of Spain’s Barraquer Institute of Barcelona after his research work in Intrasclearal cartilage grafting, becoming one of the few Australian ophthalmologists to receive this honour, and in the following year presented his final paper on Living Intrasclearal Cartilage Implants at the Inaugural Meeting of the Australian College of Ophthalmologists in Melbourne In his personal life Dr Angus was a Presbyterian and treated Sunday as a Sabbath, a day of rest. He would visit 3 or 4 country patients on a Sunday, taking his children along ‘for the ride’ and to visit with him. Sunday evenings he would play the pianola and sing Scottish songs to his family. One of Dr Angus’ patients was Margaret MacKenzie, author of a book on local shipwrecks that she’d seen as an eye witness from the late 1880’s in Peterborough, Victoria. In the early 1950’s Dr Angus, painted a picture of a shipwreck for the cover jacket of Margaret’s book, Shipwrecks and More Shipwrecks. She was blind in later life and her daughter wrote the actual book for her. Dr Angus and his wife Gladys were very involved in Warrnambool’s society with a strong interest in civic affairs. He had an interest in people and the community They were both involved in the creation of Flagstaff Hill, including the layout of the gardens. After his death (28th March 1970) his family requested his practitioner’s plate, medical instruments and some personal belongings be displayed in the Port Medical Office surgery at Flagstaff Hill Maritime Village, and be called the “W. R. Angus Collection”. The W.R. Angus Collection is significant for still being located at the site it is connected with, Doctor Angus being the last Port Medical Officer in Warrnambool. The collection of medical instruments and other equipment is culturally significant, being an historical example of medicine from late 19th to mid-20th century. Dr Angus assisted Dr Tom Ryan, a pioneer in the use of X-rays and in ocular surgery.Glass tube or cylinder with wide base and pouring lip. Measurements in ml and fl oz.flagstaff hill, warrnambool, shipwrecked-coast, flagstaff-hill, flagstaff-hill-maritime-museum, maritime-museum, shipwreck-coast, flagstaff-hill-maritime-village, measuring device, measuring cylinder, glass -
 Federation University Historical Collection
Federation University Historical CollectionCertificate, Ballarat School of Mines, William Corbould's Ballarat School of Mines Metallurgy Certificate, 11/07/1883
William Corbould was the son of a Ballarat tailor. He attended Ballarat College, and obtained certificates in assaying and metallurgy at the Ballarat School of Mines (SMB) in 1883, studying under the revered Professor Mica Smith. Corbould was not a born student and remembered his first experience at SMB: 'From the Registrar's Office I was led to be introduced to the Professor of Chemistry, one Mica Smith. The initial encounter gave me little encouragement - his large laboratory was filled with hundreds of bottles bearing strange labels with queer symbols on them. My heart sank. At the first opportunity I grabbed my hat and made for the door, but the good professor called me back. I pointed out that I was never any good at school ... so it was no use pretending to be clever enough to understand all those weird symbols! The Professor told me not to worry about that and took me to one of the benches where he found a blowpipe and a charcoal block. Mixing together two powders from bottles on the shelf he transferred a sample to the charcoal and directed the bunsen flame onto it. Soon it began to melt and a white bead appeared in front of my eyes. He then took a test tube and added a little colourless liquid from each of two bottles. A beautiful dark blue colour appeared. My interest was won.' During Corbould's mining career he travelled to Europe twice, and visited most of Australia's main mining fields. Corbould started his career as an assayer at Pinnacle Silver Mine, Silverton, and was then a self-employed assayer at Broken Hill. Corbould became an assayer for the infant BHP mine, and later worked in Kalgoorlie and Coolgardie, including managing Hannan's Reward, the oldest gold mine on the Kalgoorlie gold field. He spent 13 years at the Mount Elliott copper fields as general manager. In 1923, at the age of 57, Corbould went to Mount Isa and reported on options, experimented with new metallurgical processes and floated a company. John Carden of CRA said: 'Corbould was the man who brought Urquhart to Mount Isa. He was the man who made it all possible. He is tremendously important in the Mount Isa story, because he was the first technical man, the first professional man on the scene. He was responsible as I said, for bringing finance to the place, but I think even more importantly he was the first man to recognise the need to put all the little claims in the Mount Isa discovery together. I think perhaps his major contribution to Mount Isa was this amalgamation on the various claims. He recognised that the ore bodies at Mount Isa were not as rich as Broken Hill and they would never have survived had it been fragmented, so he was terribly important.' After completing major financial negotiations for Mt Isa Mine from London in 1927 Corbould remained in Europe where he remained until his death. Corbould was awarded the Legion of Honour of the American Institute of Mining and Metallurigical Engineers for fifty years service. Corbould died at Monaco in 1949 at the age of 82. (http://guerin.ballarat.edu.au/curator/honour-roll/honourroll_Corbould,William.shtml)A white paper certificate with black printed and handwritten text, and a blue Ballarat School of Mines seal. The certificate is signed by Andrew Berry (Registrar) and James Oddie (Vice-President).Signed on the left 'W.H. Corbould'mining, ballarat school of mines, mining alumni, metallurgy, james oddie, andrew berry, william corbould, corbould, berry, oddie -
 Bendigo Historical Society Inc.
Bendigo Historical Society Inc.Administrative record - Abbott Collection: Jan to Jun 1893: records and receipts for purchases by J.H. Abbott & Co
various 1893 company letterhead receipts2403.86 (A to G) Abbott Collection: Jan to Jun 1893: records and receipts for purchases by J.H. Abbott & Co. from a wide range of generally Melbourne based companies 2043.86A 40 records held by J.H. Abbott & Co. Jan to Jun 1893: for purchases (or sales) 2403.86B 10 receipts for purchases by J.H. Abbott & Co. from Thomas P. Power, Saddlers, Ironmongers & Manufacturers398-400 Little Bourke St Melbourne 2403.86C 10 receipts for purchases by J.H. Abbott from The India Rubber, Gutta Percha & Telegraph Works Co. 106 Cannon St London 2403.86D 4 receipts for purchases by J.H. Abbott & Co. from Cashel, Baxter & Co., 508 Collins Str Melbourne 2403.86E 7 receipts for purchases by J.H. Abbott from Ullathorne & Co., 269 Lonsdale St Melbourne 2403.86F 5 receipts for purchases by J.H. Abbott from Michaelis, Hallenstein & Co, Tanners, Curriers & Leather Merchants, Importers of Grindery, 382-384 Lonsdale St Melbourne 2403.86G 14 receipts for purchases by J.H. Abbott from a variety of companies: Nobel's Hamburg Dynamite Co. Ltd. - gelignite Thomas Mitchell, Paint & General Brush Manufacturers357 Lonsdale St Melbourne R.M. Watson & Co. 345 Flinders Lane Melbourne; Paper Makers, Agents, Stationers and Importers (two receipts) Wm Dodgshun & Sons, 258 &260 Flinders Lane, East Melbourne; Importers & Warehousemen (two receipts) The New Zealand Loan & Mercantile Agency Company Limited, Collins Street West, Melbourne; Melbourne Wool & Grain Warehouses Walter H. Carwardine, Bendigo Soap, Soda Crystal & candle Works, near the Municipal Cattle Yards J. Kitchen & Sons & Apollo Company; 326 Flinders Lane, Melbourne J. Kennon & Sons, Tanners, Curriers & Leather Merchants; Tannery, River St., near Hawthorn Bridge Innes - Noad V. Halfden (!!!), Tea Merchants & Importers; 201 1to 205 William Street, Melbourne The Indian Company, Lubricating Oil Merchants; Normanby Road, South Melbourne; 375 Flinders Lane, corner Queen Street, Melbourne A. Spooner Manufacturer of Improved Harness Composition, Improved Black Oil, Harness Liquid, Waterproof Harness Blacking, saddle Soap, Boot Top Powder (all colours), Polishing Cream, Breeches Paste, Universal Cream and Embrocations. Australian Asbestos Mfg Co. 266 Flinders St Easttrade company purchases 1893 -
 Parks Victoria - Wilsons Promontory Lightstation
Parks Victoria - Wilsons Promontory LightstationTank lid
Lid for ship's tanks used for early domestic water storage (1860's) at the lightstation The water tank and lid are probably from the same unit that was used for transporting drinking water or perishable dry goods on ships. The unit comprised a large, riveted metal tank which was fitted with a heavy cast iron round lid to form a hermetically sealed container. It had a rubber sealing ring ‘which was screwed tight with the aid of lugs cast into the lid and wedges cast into the rim of the loading hole’. A raised iron rod welded across the outer face of many lids allowed for screwing the lid tight. Ship tanks were invented in1808 by notable engineer, Richard Trevithick and his associate John Dickinson. Their patent obtained the same year described the tank’s superior cubic shape that allowed it to fit squarely as a container in ships and thus use space efficiently, while its metal fabric preserved and secured its contents, whether liquid or solid, from damage. The containers revolutionised the movement of goods by ship and made wooden casks redundant. Research by Michael Pearson has determined that they were carried on passages to Australia from at least the 1830s, conveying ships’ victuals and water storage as well as general goods heading for the colonies, and by the 1870s they were in common use. Once in the colonies, the tanks were often recycled and adapted for many resourceful uses such as water tanks, packing cases, dog kennels, oil containers and food stores and this invariably led to the separation of the lid and tank. Raised lettering on the lids indicates that nearly all of the ship tanks transported to Australia came from London manufacturers, and it was usual also for the brand name to feature as a stencil on the associated square tank but in most cases this eventually wore off. It is not known if the Wilsons Promontory tank retains its stencil, and the heavy lid will need to be turned over to reveal its manufacturer’s name. How it came to the lightstation is also not known, but it was either brought to the site as a recycled tank or salvaged from a shipwreck. Pearson writes that Ship tanks show up at a wide range of sites, many of them isolated like lighthouses. They were, I think, usually taken there for the purposes they filled, usually water storage, as they were readily available, relatively light to transport, and probably very cheap to buy as second‐hand goods containers. In rural areas they may have been scavenged for their new uses from local stores, to whom goods were delivered in them. Recycled to serve as a water tank, the Wilsons Promontory tank is the last surviving example of several that were used at the site to hold water for domestic consumption. The tank has had its lid removed and a tap fitted to the one of the sides. It stands on concrete blocks next to a building to receive water running off the roof via a metal pipe. Wilsons Promontory is the only lightstation managed by Parks Victoria with a tank container, although Cape Otway and Point Hicks have lids. Parks Victoria has identified four other lids which include two at Point Hicks, one manufactured by Lancaster and Co. the other by Bellamy. Cape Otway also has two, one unidentified and the other by the Bow Tank Works, East London, which produced tanks between 1910 and 1930. Pearson notes that ‘surviving lids are far less numerous than the tanks themselves, presumably because the uses to which the tanks were put did not require the lid to be retained’. The tank and lid, which are possibly part of the same unit, have first level contributory significance for their historic values and rarity. Round ship's tanks lid, iron. -
 Federation University Historical Collection
Federation University Historical CollectionPhotograph, Ballarat School of Mines Corbould Building
Corbould Hall was named after Ballarat School of Mines alumni WIlliam Corbould. William Corbould was the son of a Ballarat tailor. He attended Ballarat College, and obtained certificates in assaying and metallurgy at the Ballarat School of Mines (SMB) in 1883, studying under the revered Professor Mica Smith. Corbould was not a born student and remembered his first experience at SMB: 'From the Registrar's Office I was led to be introduced to the Professor of Chemistry, one Mica Smith. The initial encounter gave me little encouragement - his large laboratory was filled with hundreds of bottles bearing strange labels with queer symbols on them. My heart sank. At the first opportunity I grabbed my hat and made for the door, but the good professor called me back. I pointed out that I was never any good at school ... so it was no use pretending to be clever enough to understand all those weird symbols! The Professor told me not to worry about that and took me to one of the benches where he found a blowpipe and a charcoal block. Mixing together two powders from bottles on the shelf he transferred a sample to the charcoal and directed the bunsen flame onto it. Soon it began to melt and a white bead appeared in front of my eyes. He then took a test tube and added a little colourless liquid from each of two bottles. A beautiful dark blue colour appeared. My interest was won.' During Corbould's mining career he travelled to Europe twice, and visited most of Australia's main mining fields. Corbould started his career as an assayer at Pinnacle Silver Mine, Silverton, and was then a self-employed assayer at Broken Hill. Corbould became an assayer for the infant BHP mine, and later worked in Kalgoorlie and Coolgardie, including managing Hannan's Reward, the oldest gold mine on the Kalgoorlie gold field. He spent 13 years at the Mount Elliott copper fields as general manager. In 1923, at the age of 57, Corbould went to Mount Isa and reported on options, experimented with new metallurgical processes and floated a company. John Carden of CRA said: 'Corbould was the man who brought Urquhart to Mount Isa. He was the man who made it all possible. He is tremendously important in the Mount Isa story, because he was the first technical man, the first professional man on the scene. He was responsible as I said, for bringing finance to the place, but I think even more importantly he was the first man to recognise the need to put all the little claims in the Mount Isa discovery together. I think perhaps his major contribution to Mount Isa was this amalgamation on the various claims. He recognised that the ore bodies at Mount Isa were not as rich as Broken Hill and they would never have survived had it been fragmented, so he was terribly important.' After completing major financial negotiations for Mt Isa Mine from London in 1927 Corbould remained in Europe where he remained until his death. Corbould was awarded the Legion of Honour of the American Institute of Mining and Metallurigical Engineers for fifty years service. Corbould died at Monaco in 1949 at the age of 82. He bequested 6000 pounds to the Ballarat School of Mines, his will stating 'for the purpose of founding a scholarship to commemorate the memory of the late Alfred Mica Smith'. The accumulated income from this sum provides the Mica Smith travelling scholarship, enabling successful students in mining, metallurgy or chemistry to undertake a year's travelling abroad. The first award was made in 1957. In the same year a general purpose hall at SMB was named the Corbould Hall as a tribute to a distinguished former student and generous benefactor.ballarat school of mines corbould building, corbould hall, corbould building -
 Flagstaff Hill Maritime Museum and Village
Flagstaff Hill Maritime Museum and VillageContainer - Ink Bottle and Pen, Caldwell’s Ink Factory, Early 20th century
This shaped ink bottle made by Caldwell's is called a 'boat ink bottle'. It was shaped especially to hold a nib pen when the pen was not in use. The design of the bottle is sometimes called a ‘cottage’ or ‘boat’ shape. The Caldwell’s handmade glass ink bottle was mouth-blown into a two-piece mould, a method often used in the mid-to-late 19th century. The glass blower burst the bottle off the end of his blowpipe with a tool, leaving an uneven mouth and sharp edge on the bottle, which was usually filed. The bottle was then filled with ink and sealed with a cork. More expensive bottles would have a lip added, which was more time-consuming and costly to produce. The capacity for a bottle such as this was about 3 ½ oz (ounces) equal to about 100 ml. Pen and ink have been in use for handwriting since about the seventh century. A quill pen made from a bird’s feather was used up until around the mid-19th century. In the 1850s a steel point nib for the dip pen was invented and could be manufactured on machines in large quantities. The nis only held a small amount of ink so users had to frequently dip the nib into an ink well for more ink. Handwriting left wet ink on the paper, so the blotting paper was carefully used to absorb the excess ink and prevent smudging. Ink could be purchased as a ready-to-use liquid or in powdered form, which needed to be mixed with water. In the 1880s a successful, portable fountain pen gave smooth-flowing ink and was easy to use. In the mid-20th century, the modern ballpoint pen was readily available and inexpensive, so the fountain pen lost its popularity. However, artisans continue to use nib pens to create beautiful calligraphy. Caldwell’s Ink Co. – F.R. Caldwell established Caldwell’s Ink Company in Australia around 1902. In Victoria, he operated from a factory at Victoria Avenue, Albert Park, until about 1911, then from Yarra Bank Road in South Melbourne. Newspaper offices were appointed as agencies to sell his inks, for example, in 1904 the New Zealand Evening Star sold Caldwell’s Flo-Eesi blue black ink in various bottle sizes, and Murchison Advocate (Victoria) stocked Caldwell’s ink in crimson, green, blue black, violet, and blue. Caldwell’s ink was stated to be “non-corrosive and unaffected by steel pens”. A motto used in advertising in 1904-1908 reads ‘Makes Writing a Pleasure’. Stationers stocked Caldwell’s products and hawkers sold Caldwell’s ink stands from door to door in Sydney in the 1910s and 1920s. In 1911 Caldwell promised cash for returned ink bottles and warned of prosecution for anyone found refilling his bottles. Caldwell’s Ink Stands were given as gifts. The company encouraged all forms of writing with their Australian-made Flo-Eesi writing inks and bottles at their impressive booth in the ‘All Australian Exhibition’ in 1913. It advertised its other products, which included Caldwell’s Gum, Caldwell’s Stencil Ink (copy ink) and Caldwell’s Quicksticker as well as Caldwell’s ‘Zac’ Cough Mixture. Caldwell stated in a 1920 article that his inks were made from a formula that was over a century old, and were scientifically tested and quality controlled. The formula included gallic and tannic acids and high-quality dyes to ensure that they did not fade. They were “free from all injurious chemicals”. The permanent quality of the ink was important for legal reasons, particularly to banks, accountants, commerce, municipal councils and lawyers. The Caldwell’s Ink Company also exported crates of its ink bottles and ink stands overseas. Newspaper advertisements can be found for Caldwell’s Ink Company up until 1934 when the company said they were the Best in the business for 40 years.This pen and ink bottle set is of significance as the bottle has its original cork and retains remnants of ink, which was made from a recipe that at the time was over 100 years old, according to Caldwell.. The handmade, mould blown method of manufacture is representative of a 19th-century handcraft industry that is now been largely replaced by mass production. The bottle and its contents are of state significance for being produced by an early Melbourne industry and exported overseas. The pen and ink set is historically significant as it represents methods of handwritten communication that were still common up until the mid-20th century when fountain pens and modern ballpoint pens became popular and convenient and typewriters were becoming part of standard office equipment.Victorian boat ink bottle; small rectangular clear glass ink bottle with horizontal grooves made in the glass for resting and holding the pen. The set includes one pen and nib with the bottle and cork. The bottle is made by Caldwell's and contains its Flo-Eesi Blue Black Ink brand."Caldwell's Flo-Eesi Blue Black Ink."flagstaff hill, warrnambool, maritime museum, maritime village, great ocean road, shipwreck coast, ink, nib pen, writing ink, writing, copying, banks, lawyers, commerce, student, permanent ink, flo-eesi, blue black ink, stationery, record keeping, handwriting, writing equipment, writing accessory, office supply, cottage bottle, boat bottle, mouth-blown bottle, two-part mould, sheer-lip bottle, burst-lip, cork seal, f r caldwell, caldwell’s ink company, albert park, south melbourne, inkstands, stencil ink, copy ink, quicksticker, zac cough mixture -
 Flagstaff Hill Maritime Museum and Village
Flagstaff Hill Maritime Museum and VillageContainer - Ink Bottles, Caldwell’s Ink Factory, Early 20th century
This crate of bottles may have come from a wholesaler, business, stationer or school. The design of the bottles is sometimes called a ‘cottage’ or ‘boat’ shape. Each of the 70 Caldwell’s handmade glass ink bottles was mouth-blown into a two-piece mould, a method often used in the mid-to-late 19th century. The glass blower burst the bottle off the end of his blowpipe with a tool, leaving an uneven mouth and sharp edge on the bottle, which was usually filed. The bottle was then filled with ink and sealed with a cork. More expensive bottles would have a lip added, which was more time-consuming and costly to produce. The capacity for a bottle such as this was about 3 ½ oz (ounces) equal to about 100 ml. Pen and ink have been in use for handwriting since about the seventh century. A quill pen made from a bird’s feather was used up until around the mid-19th century. In the 1850s a steel point nib for the dip pen was invented and could be manufactured on machines in large quantities. The nis only held a small amount of ink so users had to frequently dip the nib into an ink well for more ink. Handwriting left wet ink on the paper, so the blotting paper was carefully used to absorb the excess ink and prevent smudging. Ink could be purchased as a ready-to-use liquid or in powdered form, which needed to be mixed with water. In the 1880s a successful, portable fountain pen gave smooth-flowing ink and was easy to use. In the mid-20th century, the modern ballpoint pen was readily available and inexpensive, so the fountain pen lost its popularity. However, artisans continue to use nib pens to create beautiful calligraphy. Caldwell’s Ink Co. – F.R. Caldwell established Caldwell’s Ink Company in Australia around 1902. In Victoria, he operated from a factory at Victoria Avenue, Albert Park, until about 1911, then from Yarra Bank Road in South Melbourne. Newspaper offices were appointed as agencies to sell his inks, for example, in 1904 the New Zealand Evening Star sold Caldwell’s Flo-Eesi blue black ink in various bottle sizes, and Murchison Advocate (Victoria) stocked Caldwell’s ink in crimson, green, blue black, violet, and blue. Caldwell’s ink was stated to be “non-corrosive and unaffected by steel pens”. A motto used in advertising in 1904-1908 reads ‘Makes Writing a Pleasure’. Stationers stocked Caldwell’s products and hawkers sold Caldwell’s ink stands from door to door in Sydney in the 1910s and 1920s. In 1911 Caldwell promised cash for returned ink bottles and warned of prosecution for anyone found refilling his bottles. Caldwell’s Ink Stands were given as gifts. The company encouraged all forms of writing with their Australian-made Flo-Eesi writing inks and bottles at their impressive booth in the ‘All Australian Exhibition’ in 1913. It advertised its other products, which included Caldwell’s Gum, Caldwell’s Stencil Ink (copy ink) and Caldwell’s Quicksticker as well as Caldwell’s ‘Zac’ Cough Mixture. Caldwell stated in a 1920 article that his inks were made from a formula that was over a century old, and were scientifically tested and quality controlled. The formula included gallic and tannic acids and high-quality dyes to ensure that they did not fade. They were “free from all injurious chemicals”. The permanent quality of the ink was important for legal reasons, particularly to banks, accountants, commerce, municipal councils and lawyers. The Caldwell’s Ink Company also exported crates of its ink bottles and ink stands overseas. Newspaper advertisements can be found for Caldwell’s Ink Company up until 1934 when the company said they were the Best in the business for 40 years.This large collection of similar ink bottles is of particular significance as the bottles have come from the same source, most have their original corks and some retain their original labels, which is rare. The method of manufacture of these bottles is also representative of a 19th-century handcraft industry that is now been largely replaced by mass production. The bottles and their contents are of state significance for being produced by an early Melbourne industry and exported overseas. This case of ink bottles is historically significant as it represents methods of handwritten communication that were still common up until the mid-20th century when fountain pens and modern ballpoint pens became popular and convenient and typewriters were becoming part of standard office equipment.Ink bottles in a wooden crate; 70 rectangular, hand-blown clear glass ink bottles. They have side seams, uneven thickness, especially at the bases, and rough, burst-off mouths. The shoulders on the long sides have horizontal grooves used for pen rests. The bottles vary; some have labels, some contain remnants of blue-black ink, and many have their original corks. The glass has bubbles and imperfections. The remnants of printed labels are on white paper with a swirly border and black text. The bottles contained Caldwell’s blend of blue black ‘Flo-Eesi’ ink.Printed on label; “CALDWELL FLO-EESI BLUE BLACK INK” “ - - - - “ Printed script signature “F.R. Caldwell”flagstaff hill, warrnambool, maritime village, maritime museum, shipwreck coast, great ocean road, ink, nib pen, writing ink, writing, copying, banks, lawyers, commerce, student, permanent ink, flo-eesi, blue black ink, stationery, record keeping, handwriting, writing equipment, writing accessory, office supply, cottage bottle, boat bottle, mouth-blown bottle, two-part mould, sheer-lip bottle, burst-lip, cork seal, f r caldwell, caldwell’s ink company, albert park, south melbourne, inkstands, stencil ink, copy ink, quicksticker, zac cough mixture -
 NMIT (Northern Melbourne Institute of TAFE)
NMIT (Northern Melbourne Institute of TAFE)DVDs: Promotional DVDs NMIT 1990-2010
Instructional and promotional DVDs ranging in date from 1990-2010 promoting courses and services of NMIT. 1990s An Introduction to NMIT 1996 X 2 Building & Construction Heidelberg 1992 Building & Construction Heidelberg 1992 1, 2, 3 (Umatic) Concrete pour - Heidelberg 1992 Greensborough Music Promotional 1994 Making the move 1996 (also booklet) NMCOT College Promotion 1990 NMCOT College promotion 1992 NMCOT Corporate Video 1992 NMCOT Corporate video 1994 NMCOT Enrolment form 1991 (Umatic) NMCOT Enrolment Form 1992 NMCOT To Market to Market Promotional video 1993 NMCOT To Market to Market Promotional video 1994 NMIT School of Arts & Social Sciences 1996 NMIT School of Building & Construction 1996 NMIT School of Business 1996 NMIT School of Electrical, Electronics & Sciences 1996 NMIT School of Horticulture & Rural Studies 1995 NMIT School of Horticulture & Rural Studies 1996 NMIT School of Manufacturing Engineering 1996 NMIT School of Mechanical Manufacturing 1996 NMIT School of Tourism & Hospitality 1996 NMIT Faculty of Earch Sciences 1998 NMIT The Electrical Connection 1995 Open Day 1992 Student Information 2003-2008 Advanced Diploma of Music Performance (undated) Challenges accepted, NMIT Roadshow 2005 Mechanical manufacturing 2003 Promotional video (master) 2006 Songwriting competition NMIT 2008 The Electrical connection 2003 2009 Animal Studies 2009 Bachelor of Viticulture & winemaking 2009 Certificate III in Aged Care 2009 Children’s Services 2009 Courses through Design Drafting & Interior Fittings 2009 X2 Equine Studies 2009 (also accompanying book) Erection and Dismantling procedure for an Oldfields Mobile Scaffold 2009 Facilitate Individual Learning Activity The REV shop Case Study 2009 Formwork to Columns and Beams 2009 Green skills Centre of Excellence : Contributing to sustainability directly through the design, our actions and by educating future generations on sustainable technology, [DVD], NMIT Epping Campus, [2009] Interior design 2009 Locksmith Apprenticeships 2009 Secondary to Tertiary: the Journey begins 2009 X 2 Visual Arts at NMIT 2009 2010 Advanced Diploma of Building design 2010 Bachelor of Accounting 2010 Bachelor of Equine Studies 2010 Certificate III in Farriery (Trade) 2010 Civil Engineering 2010 Cloisonne Enamel 2010 Conservation and Land Management 2010 Health & Community studies 2010 Horticulture 2010 How to check and adjust a single stage Liquid Petroleum Gas Regulator 2010 Locksmithing more than a trade 2010 Music, Sound & Television 2010 Pragmatic Failures in Intercultural Communication 2010 Technical Education Centre, Youth Unit, NMIT 2010 Viticulture & Winemaking 2010 Why study Community Services at NMIT 2010 courses, handbooks, nmit -
 Flagstaff Hill Maritime Museum and Village
Flagstaff Hill Maritime Museum and VillageFunctional object - Lamp Light, late 19th - early-20th century
This gas lamp light and stand came from the original manufacturer in Melbourne. Gas street lights such as this one were used in Melbourne from the mid-19th century. The lights enabled safer after-dark travel for pedestrians and vehicles and were a deterrent to crime. A lamp lighter was employed to keep the lamps lit, sometimes with little success due to weather conditions and the pranks of youths. WARRNAMBOOL Gasworks In Warrnambool prior to 1874 there were about twenty rare, individually lit street lights in Warrnambool, each with its own supply of kerosene. These lamps were in the central business area of Timor, Koroit and Liebig Streets. The Warrnambool Gas Company Ltd. was registered as an incorporated company in 1873. It was a private, locally owned business. It was located at 209-215 Merri Street, Warrnambool, on the land, which is just west of the later-built railway station. The first managers of the Gas Company lived in a substantial stone house on site, but later the managers lived in a residence in Henna Street between Merri and Timor Streets. The original home, which still stands, became a residence for the Railway Station Master from about 1890. In August 1874 the construction of the gasworks was complete and at the end of that month gas was supplied to all of the existing lamps in Warrnambool for the first time. The Warrnambool Gas Company wound up in 1880-1881 and was purchased by the Warrnambool Borough Council with money raised by a loan – the Borough’s first ‘loan transaction’. The Council established a piped network to supply gas to other street connections. The gasworks were privatised and upgraded in 1952. In 1972 the town supply was converted to liquid petroleum gas and by the early 1980s the gasworks were closed down. In 1986 Warrnambool was supplied with natural gas from a site near Port Campbell. The Warrnambool gasworks supplied all street and shop lighting and most domestic lighting until 1923 when electricity was available for lighting. Bromfield Street in Warrnambool was named after the director of the gasworks, James Astley Bromfield (1823-1903). He arrived in Warrnambool from Worcestershire, England, in 1852 and was very active in the local council and community. Cockman Street was named after the first secretary of the gasworks in 1874, Walter Cockman (c.1821-1892). He was a Mayor and businessman. The second Manager, Luther Rodgers, worked for the gas company for about twenty years and both Rodger Place and Rodgers Road in Warrnambool have been named after him. LAMP LIGHTS IN MELBOURNE In the 1820s Melbourne's innkeepers were legally required to have a lamp light outside their premises from sunset to sunrise. This was the first instance of street lamps being used in Melbourne. In 1847 the first oil lamp was used in the city. In 1849 a gas lamp was installed on the Swanston Street Bridge and much of the city had oil lamps installed by then. In August 1857 the installation of street gas lamps began in Melbourne. They were welcomed for the much brighter illumination they gave. By 1860 there were 414 lamp pillars. The phrase was quoted often - "A light was as good as a policeman". The first gas burners used for street lighting were called 'fishtail' gas burners. These were replaced in the early 1900s by gas mantles. The City of Melbourne Gas Coke Company was formed in 1850 but due to the Gold Rush the manufacture and distribution of the gas supply was delayed until January 1856. By the 1890s the gas supplying the lights was supplied by three companies in Melbourne. In 1879 a football match was played at the MCG under electric lighting and gradually electric arc lights were installed inside and outside buildings in the city. Lamp lights such as the one in Flagstaff Hill’s collection were no longer needed. (References: John Lindsay re Lamp Light history 2019-01-29, Former Warrnambool Gas Company Limited, Victorian Heritage Database Report, Heritage Number 149746 https://vhd.heritagecouncil.vic.gov.au/places/149746/download-report ) The lamp light is representative of the lamps used in Melbourne from the mid-nineteenth century to light the streets at night and make Melbourne a safer city. The lamp is also representative of the gas street lighting in Warrnambool from the mid-1870s-1920s.Lamp light or gas light. Street light, one of the last gas street lights removed from Melbourne. (Reconditioned by Friends of Flagstaff Hill, 2013)flagstaff hill, warrnambool, shipwrecked coast, flagstaff hill maritime museum, maritime museum, shipwreck coast, flagstaff hill maritime village, great ocean road, lamp light, gas light, gas lamp, street lamp, street light, gas street light, melbourne street lighting, warrnambool street lighting, melbourne gas street light, warrnambool gas company, warrnambool gasworks, james bromfield, walter cockman, luther rodgers, city of melbourne gas coke company -
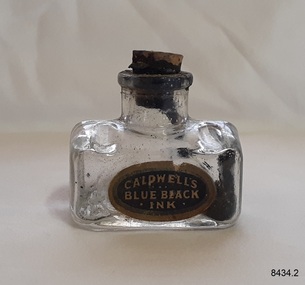 Flagstaff Hill Maritime Museum and Village
Flagstaff Hill Maritime Museum and VillageContainer - Ink Bottle, Caldwell’s Ink Factory, Late 19th to early 20th centuries
This design of the bottle is sometimes called a ‘cottage’ or ‘boat’ shape. The Caldwell’s handmade glass ink bottle was mouth-blown into a three-piece mould, a method often used in the late 19th and early 20th centuries, with the maker's name engraved into the mould section for the base. The glass blower would cut the bottle off the end of his blowpipe with a tool and join a mouth onto the top, rolling the lip. The bottle was then filled with ink and sealed with a cork. This method of manufacture was more time-consuming and costly to produce than those made in a simple two-piece mould and 'cracked' off the blowpipe. The capacity for a bottle such as this was about 3 ½ oz (ounces) equal to about 100 ml. This particular bottle is unusual as it has four sloping indents at the corners of the shoulder, most likely for resting a pen with its nib upwards and the handle resting on a flat surface. Most of the bottles made during this era had horizontal pen rests that were indented into both of the long sides of the shoulder. Pen and ink have been in use for handwriting since about the seventh century. A quill pen made from a bird’s feather was used up until around the mid-19th century. In the 1850s a steel point nib for the dip pen was invented and could be manufactured on machines in large quantities. This only held a small amount of ink so users had to frequently dip the nib into an ink well for more ink. Handwriting left wet ink on the paper, so the blotting paper was carefully used to absorb the excess ink and prevent smudging. Ink could be purchased as a ready-to-use liquid or in powdered form, which needed to be mixed with water. In the 1880s a successful, portable fountain pen gave smooth-flowing ink and was easy to use. In the mid-20th century, the modern ballpoint pen was readily available and inexpensive, so the fountain pen lost its popularity. However, artisans continue to use nib pens to create beautiful calligraphy. Caldwell’s Ink Co. – F.R. Caldwell established Caldwell’s Ink Company in Australia around 1902. In Victoria, he operated from a factory at Victoria Avenue, Albert Park, until about 1911, then from Yarra Bank Road in South Melbourne. Newspaper offices were appointed as agencies to sell his inks, for example, in 1904 the New Zealand Evening Star sold Caldwell’s Flo-Eesi blue black ink in various bottle sizes, and Murchison Advocate (Victoria) stocked Caldwell’s ink in crimson, green, blue black, violet, and blue. Caldwell’s ink was stated to be “non-corrosive and unaffected by steel pens”. A motto used in advertising in 1904-1908 reads ‘Makes Writing a Pleasure’. Stationers stocked Caldwell’s products and hawkers sold Caldwell’s ink stands from door to door in Sydney in the 1910s and 1920s. In 1911 Caldwell promised cash for returned ink bottles and warned of prosecution for anyone found refilling his bottles. Caldwell’s Ink Stands were given as gifts. The company encouraged all forms of writing with their Australian-made Flo-Eesi writing inks and bottles at their impressive booth in the ‘All Australian Exhibition’ in 1913. It advertised its other products, which included Caldwell’s Gum, Caldwell’s Stencil Ink (copy ink) and Caldwell’s Quicksticker as well as Caldwell’s ‘Zac’ Cough Mixture. Caldwell stated in a 1920 article that his inks were made from a formula that was over a century old, and were scientifically tested and quality controlled. The formula included gallic and tannic acids and high-quality dyes to ensure that they did not fade. They were “free from all injurious chemicals”. The permanent quality of the ink was important for legal reasons, particularly to banks, accountants, commerce, municipal councils and lawyers. The Caldwell’s Ink Company also exported crates of its ink bottles and ink stands overseas. Newspaper advertisements can be found for Caldwell’s Ink Company up until 1934 when the company said they were the Best in the business for 40 years.This hand-blown bottle is significant for being the only bottle in our collection with the unusual sloping pen rests on its shoulder. It is also significant for being made in a less common three-piece mould. The method of manufacture is representative of a 19th-century handcraft industry that is now been largely replaced by mass production. The bottle is of state significance for being produced by an early Melbourne industry and exported overseas. This ink bottle is historically significant as it represents methods of handwritten communication that were still common up until the mid-20th century when fountain pens and modern ballpoint pens became popular and convenient and typewriters were becoming part of standard office equipment.Ink bottle; rectangular base, hand-blown clear glass bottle with its own cork. The bottle has side seams from the base to the mouth, an indented base and an applied lip. The corners of the shoulder sides have unusual diagonal grooves that slope down and outwards that may have been used as pen rests. Inside the bottle are remnants of dried blue-black ink. The glass has imperfections and some ripples on the surface. The bottle has an attached oval black label label with gold-brown printed text and border. The base has an embossed inscription. The bottles once contained Caldwell’s blend of blue black ink.Printed on label; “CALDWELL's BLUE BLACK INK” Embossed on the base "CALDWELLS"flagstaff hill, warrnambool, maritime village, maritime museum, shipwreck coast, great ocean road, ink, nib pen, writing ink, writing, copying, banks, lawyers, commerce, student, permanent ink, blue black ink, stationery, record keeping, handwriting, writing equipment, writing accessory, office supply, cottage bottle, boat bottle, mouth-blown bottle, cork seal, f r caldwell, caldwell’s ink company, albert park, south melbourne, inkstands, stencil ink, copy ink, quicksticker, zac cough mixture, three part mould, cauldwells, cauldwell's -
 Flagstaff Hill Maritime Museum and Village
Flagstaff Hill Maritime Museum and VillageAnimal specimen - Whale bone, Undetermined
Prior to carrying out a detailed condition report of the cetacean skeletons, it is useful to have an understanding of the materials we are likely to encounter, in terms of structure and chemistry. This entry invites you to join in learning about the composition of whale bone and oil. Whale bone (Cetacean) bone is comprised of a composite structure of both an inorganic matrix of mainly hydroxylapatite (a calcium phosphate mineral), providing strength and rigidity, as well as an organic protein ‘scaffolding’ of mainly collagen, facilitating growth and repair (O’Connor 2008, CCI 2010). Collagen is also the structural protein component in cartilage between the whale vertebrae and attached to the fins of both the Killer Whale and the Dolphin. Relative proportions in the bone composition (affecting density), are linked with the feeding habits and mechanical stresses typically endured by bones of particular whale types. A Sperm Whale (Physeter macrocephalus Linnaeus, 1758) skeleton (toothed) thus has a higher mineral value (~67%) than a Fin Whale (Balaenoptera physalus Linnaeus, 1758) (baleen) (~60%) (Turner Walker 2012). The internal structure of bone can be divided into compact and cancellous bone. In whales, load-bearing structures such as mandibles and upper limb bones (e.g. humerus, sternum) are largely composed of compact bone (Turner Walker 2012). This consists of lamella concentrically deposited around the longitudinal axis and is permeated by fluid carrying channels (O’Connor 2008). Cancellous (spongy) bone, with a highly porous angular network of trabeculae, is less stiff and thus found in whale ribs and vertebrae (Turner Walker 2012). Whale oil Whales not only carry a thick layer of fat (blubber) in the soft tissue of their body for heat insulation and as a food store while they are alive, but also hold large oil (lipid) reserves in their porous bones. Following maceration of the whale skeleton after death to remove the soft tissue, the bones retain a high lipid content (Higgs et. al 2010). Particularly bones with a spongy (porous) structure have a high capacity to hold oil-rich marrow. Comparative data of various whale species suggests the skull, particularly the cranium and mandible bones are particularly oil rich. Along the vertebral column, the lipid content is reduced, particularly in the thoracic vertebrae (~10-25%), yet greatly increases from the lumbar to the caudal vertebrae (~40-55%). The chest area (scapula, sternum and ribs) show a mid-range lipid content (~15-30%), with vertically orientated ribs being more heavily soaked lower down (Turner Walker 2012, Higgs et. al 2010). Whale oil is largely composed of triglycerides (molecules of fatty acids attached to a glycerol molecule). In Arctic whales a higher proportion of unsaturated, versus saturated fatty acids make up the lipid. Unsaturated fatty acids (with double or triple carbon bonds causing chain kinks, preventing close packing (solidifying) of molecules), are more likely to be liquid (oil), versus solid (fat) at room temperature (Smith and March 2007). Objects Made From the Whaling Industry We all know that men set forth in sailing ships and risked their lives to harpoon whales on the open seas throughout the 1800s. And while Moby Dick and other tales have made whaling stories immortal, people today generally don't appreciate that the whalers were part of a well-organized industry. The ships that set out from ports in New England roamed as far as the Pacific in hunt of specific species of whales. Adventure may have been the draw for some whalers, but for the captains who owned whaling ships, and the investors which financed voyages, there was a considerable monetary payoff. The gigantic carcasses of whales were chopped and boiled down and turned into products such as the fine oil needed to lubricate increasing advanced machine tools. And beyond the oil derived from whales, even their bones, in an era before the invention of plastic, was used to make a wide variety of consumer goods. In short, whales were a valuable natural resource the same as wood, minerals, or petroleum we now pump from the ground. Oil From Whale’s Blubber Oil was the main product sought from whales, and it was used to lubricate machinery and to provide illumination by burning it in lamps. When a whale was killed, it was towed to the ship and its blubber, the thick insulating fat under its skin, would be peeled and cut from its carcass in a process known as “flensing.” The blubber was minced into chunks and boiled in large vats on board the whaling ship, producing oil. The oil taken from whale blubber was packaged in casks and transported back to the whaling ship’s home port (such as New Bedford, Massachusetts, the busiest American whaling port in the mid-1800s). From the ports it would be sold and transported across the country and would find its way into a huge variety of products. Whale oil, in addition to be used for lubrication and illumination, was also used to manufacture soaps, paint, and varnish. Whale oil was also utilized in some processes used to manufacture textiles and rope. Spermaceti, a Highly Regarded Oil A peculiar oil found in the head of the sperm whale, spermaceti, was highly prized. The oil was waxy, and was commonly used in making candles. In fact, candles made of spermaceti were considered the best in the world, producing a bright clear flame without an excess of smoke. Spermaceti was also used, distilled in liquid form, as an oil to fuel lamps. The main American whaling port, New Bedford, Massachusetts, was thus known as "The City That Lit the World." When John Adams was the ambassador to Great Britain before serving as president he recorded in his diary a conversation about spermaceti he had with the British Prime Minister William Pitt. Adams, keen to promote the New England whaling industry, was trying to convince the British to import spermaceti sold by American whalers, which the British could use to fuel street lamps. The British were not interested. In his diary, Adams wrote that he told Pitt, “the fat of the spermaceti whale gives the clearest and most beautiful flame of any substance that is known in nature, and we are surprised you prefer darkness, and consequent robberies, burglaries, and murders in your streets to receiving as a remittance our spermaceti oil.” Despite the failed sales pitch John Adams made in the late 1700s, the American whaling industry boomed in the early to mid-1800s. And spermaceti was a major component of that success. Spermaceti could be refined into a lubricant that was ideal for precision machinery. The machine tools that made the growth of industry possible in the United States were lubricated, and essentially made possible, by oil derived from spermaceti. Baleen, or "Whalebone" The bones and teeth of various species of whales were used in a number of products, many of them common implements in a 19th century household. Whales are said to have produced “the plastic of the 1800s.” The "bone" of the whale which was most commonly used wasn’t technically a bone, it was baleen, a hard material arrayed in large plates, like gigantic combs, in the mouths of some species of whales. The purpose of the baleen is to act as a sieve, catching tiny organisms in sea water, which the whale consumes as food. As baleen was tough yet flexible, it could be used in a number of practical applications. And it became commonly known as "whalebone." Perhaps the most common use of whalebone was in the manufacture of corsets, which fashionable ladies in the 1800s wore to compress their waistlines. One typical corset advertisement from the 1800s proudly proclaims, “Real Whalebone Only Used.” Whalebone was also used for collar stays, buggy whips, and toys. Its remarkable flexibility even caused it to be used as the springs in early typewriters. The comparison to plastic is apt. Think of common items which today might be made of plastic, and it's likely that similar items in the 1800s would have been made of whalebone. Baleen whales do not have teeth. But the teeth of other whales, such as the sperm whale, would be used as ivory in such products as chess pieces, piano keys, or the handles of walking sticks. Pieces of scrimshaw, or carved whale's teeth, would probably be the best remembered use of whale's teeth. However, the carved teeth were created to pass the time on whaling voyages and were never a mass production item. Their relative rarity, of course, is why genuine pieces of 19th century scrimshaw are considered to be valuable collectibles today. Reference: McNamara, Robert. "Objects Made From the Whaling Industry." ThoughtCo, Jul. 31, 2021, thoughtco.com/products-produced-from-whales-1774070.Whale bone was an important commodity, used in corsets, collar stays, buggy whips, and toys.Whale bone in two pieces. Advanced stage of calcification as indicated by deep pitting. Off white to grey.None.flagstaff hill, warrnambool, shipwrecked-coast, flagstaff-hill, flagstaff-hill-maritime-museum, maritime-museum, shipwreck-coast, flagstaff-hill-maritime-village, whale bones, whale skeleton, whales, whale bone, corsets, toys, whips -
 Flagstaff Hill Maritime Museum and Village
Flagstaff Hill Maritime Museum and VillageAnimal specimen - Whale bone, Undetermined
Prior to carrying out a detailed condition report of the cetacean skeletons, it is useful to have an understanding of the materials we are likely to encounter, in terms of structure and chemistry. This entry invites you to join in learning about the composition of whale bone and oil. Whale bone (Cetacean) bone is comprised of a composite structure of both an inorganic matrix of mainly hydroxylapatite (a calcium phosphate mineral), providing strength and rigidity, as well as an organic protein ‘scaffolding’ of mainly collagen, facilitating growth and repair (O’Connor 2008, CCI 2010). Collagen is also the structural protein component in cartilage between the whale vertebrae and attached to the fins of both the Killer Whale and the Dolphin. Relative proportions in the bone composition (affecting density), are linked with the feeding habits and mechanical stresses typically endured by bones of particular whale types. A Sperm Whale (Physeter macrocephalus Linnaeus, 1758) skeleton (toothed) thus has a higher mineral value (~67%) than a Fin Whale (Balaenoptera physalus Linnaeus, 1758) (baleen) (~60%) (Turner Walker 2012). The internal structure of bone can be divided into compact and cancellous bone. In whales, load-bearing structures such as mandibles and upper limb bones (e.g. humerus, sternum) are largely composed of compact bone (Turner Walker 2012). This consists of lamella concentrically deposited around the longitudinal axis and is permeated by fluid carrying channels (O’Connor 2008). Cancellous (spongy) bone, with a highly porous angular network of trabeculae, is less stiff and thus found in whale ribs and vertebrae (Turner Walker 2012). Whale oil Whales not only carry a thick layer of fat (blubber) in the soft tissue of their body for heat insulation and as a food store while they are alive, but also hold large oil (lipid) reserves in their porous bones. Following maceration of the whale skeleton after death to remove the soft tissue, the bones retain a high lipid content (Higgs et. al 2010). Particularly bones with a spongy (porous) structure have a high capacity to hold oil-rich marrow. Comparative data of various whale species suggests the skull, particularly the cranium and mandible bones are particularly oil rich. Along the vertebral column, the lipid content is reduced, particularly in the thoracic vertebrae (~10-25%), yet greatly increases from the lumbar to the caudal vertebrae (~40-55%). The chest area (scapula, sternum and ribs) show a mid-range lipid content (~15-30%), with vertically orientated ribs being more heavily soaked lower down (Turner Walker 2012, Higgs et. al 2010). Whale oil is largely composed of triglycerides (molecules of fatty acids attached to a glycerol molecule). In Arctic whales a higher proportion of unsaturated, versus saturated fatty acids make up the lipid. Unsaturated fatty acids (with double or triple carbon bonds causing chain kinks, preventing close packing (solidifying) of molecules), are more likely to be liquid (oil), versus solid (fat) at room temperature (Smith and March 2007). Objects Made From the Whaling Industry We all know that men set forth in sailing ships and risked their lives to harpoon whales on the open seas throughout the 1800s. And while Moby Dick and other tales have made whaling stories immortal, people today generally don't appreciate that the whalers were part of a well-organized industry. The ships that set out from ports in New England roamed as far as the Pacific in hunt of specific species of whales. Adventure may have been the draw for some whalers, but for the captains who owned whaling ships, and the investors which financed voyages, there was a considerable monetary payoff. The gigantic carcasses of whales were chopped and boiled down and turned into products such as the fine oil needed to lubricate increasing advanced machine tools. And beyond the oil derived from whales, even their bones, in an era before the invention of plastic, was used to make a wide variety of consumer goods. In short, whales were a valuable natural resource the same as wood, minerals, or petroleum we now pump from the ground. Oil From Whale’s Blubber Oil was the main product sought from whales, and it was used to lubricate machinery and to provide illumination by burning it in lamps. When a whale was killed, it was towed to the ship and its blubber, the thick insulating fat under its skin, would be peeled and cut from its carcass in a process known as “flensing.” The blubber was minced into chunks and boiled in large vats on board the whaling ship, producing oil. The oil taken from whale blubber was packaged in casks and transported back to the whaling ship’s home port (such as New Bedford, Massachusetts, the busiest American whaling port in the mid-1800s). From the ports it would be sold and transported across the country and would find its way into a huge variety of products. Whale oil, in addition to be used for lubrication and illumination, was also used to manufacture soaps, paint, and varnish. Whale oil was also utilized in some processes used to manufacture textiles and rope. Spermaceti, a Highly Regarded Oil A peculiar oil found in the head of the sperm whale, spermaceti, was highly prized. The oil was waxy, and was commonly used in making candles. In fact, candles made of spermaceti were considered the best in the world, producing a bright clear flame without an excess of smoke. Spermaceti was also used, distilled in liquid form, as an oil to fuel lamps. The main American whaling port, New Bedford, Massachusetts, was thus known as "The City That Lit the World." When John Adams was the ambassador to Great Britain before serving as president he recorded in his diary a conversation about spermaceti he had with the British Prime Minister William Pitt. Adams, keen to promote the New England whaling industry, was trying to convince the British to import spermaceti sold by American whalers, which the British could use to fuel street lamps. The British were not interested. In his diary, Adams wrote that he told Pitt, “the fat of the spermaceti whale gives the clearest and most beautiful flame of any substance that is known in nature, and we are surprised you prefer darkness, and consequent robberies, burglaries, and murders in your streets to receiving as a remittance our spermaceti oil.” Despite the failed sales pitch John Adams made in the late 1700s, the American whaling industry boomed in the early to mid-1800s. And spermaceti was a major component of that success. Spermaceti could be refined into a lubricant that was ideal for precision machinery. The machine tools that made the growth of industry possible in the United States were lubricated, and essentially made possible, by oil derived from spermaceti. Baleen, or "Whalebone" The bones and teeth of various species of whales were used in a number of products, many of them common implements in a 19th century household. Whales are said to have produced “the plastic of the 1800s.” The "bone" of the whale which was most commonly used wasn’t technically a bone, it was baleen, a hard material arrayed in large plates, like gigantic combs, in the mouths of some species of whales. The purpose of the baleen is to act as a sieve, catching tiny organisms in sea water, which the whale consumes as food. As baleen was tough yet flexible, it could be used in a number of practical applications. And it became commonly known as "whalebone." Perhaps the most common use of whalebone was in the manufacture of corsets, which fashionable ladies in the 1800s wore to compress their waistlines. One typical corset advertisement from the 1800s proudly proclaims, “Real Whalebone Only Used.” Whalebone was also used for collar stays, buggy whips, and toys. Its remarkable flexibility even caused it to be used as the springs in early typewriters. The comparison to plastic is apt. Think of common items which today might be made of plastic, and it's likely that similar items in the 1800s would have been made of whalebone. Baleen whales do not have teeth. But the teeth of other whales, such as the sperm whale, would be used as ivory in such products as chess pieces, piano keys, or the handles of walking sticks. Pieces of scrimshaw, or carved whale's teeth, would probably be the best remembered use of whale's teeth. However, the carved teeth were created to pass the time on whaling voyages and were never a mass production item. Their relative rarity, of course, is why genuine pieces of 19th century scrimshaw are considered to be valuable collectibles today. Reference: McNamara, Robert. "Objects Made From the Whaling Industry." ThoughtCo, Jul. 31, 2021, thoughtco.com/products-produced-from-whales-1774070.Whale bone was an important commodity, used in corsets, collar stays, buggy whips, and toys.Whale bone piece. Advanced stage of calcification as indicated by deep pitting. Off white to grey.None.flagstaff hill, warrnambool, shipwrecked-coast, flagstaff-hill, flagstaff-hill-maritime-museum, maritime-museum, shipwreck-coast, flagstaff-hill-maritime-village, whales, whale bone, corsets, toys, whips -
 Flagstaff Hill Maritime Museum and Village
Flagstaff Hill Maritime Museum and VillageAnimal specimen - Whale bone, Undetermined
Prior to carrying out a detailed condition report of the cetacean skeletons, it is useful to have an understanding of the materials we are likely to encounter, in terms of structure and chemistry. This entry invites you to join in learning about the composition of whale bone and oil. Whale bone (Cetacean) bone is comprised of a composite structure of both an inorganic matrix of mainly hydroxylapatite (a calcium phosphate mineral), providing strength and rigidity, as well as an organic protein ‘scaffolding’ of mainly collagen, facilitating growth and repair (O’Connor 2008, CCI 2010). Collagen is also the structural protein component in cartilage between the whale vertebrae and attached to the fins of both the Killer Whale and the Dolphin. Relative proportions in the bone composition (affecting density), are linked with the feeding habits and mechanical stresses typically endured by bones of particular whale types. A Sperm Whale (Physeter macrocephalus Linnaeus, 1758) skeleton (toothed) thus has a higher mineral value (~67%) than a Fin Whale (Balaenoptera physalus Linnaeus, 1758) (baleen) (~60%) (Turner Walker 2012). The internal structure of bone can be divided into compact and cancellous bone. In whales, load-bearing structures such as mandibles and upper limb bones (e.g. humerus, sternum) are largely composed of compact bone (Turner Walker 2012). This consists of lamella concentrically deposited around the longitudinal axis and is permeated by fluid carrying channels (O’Connor 2008). Cancellous (spongy) bone, with a highly porous angular network of trabeculae, is less stiff and thus found in whale ribs and vertebrae (Turner Walker 2012). Whale oil Whales not only carry a thick layer of fat (blubber) in the soft tissue of their body for heat insulation and as a food store while they are alive, but also hold large oil (lipid) reserves in their porous bones. Following maceration of the whale skeleton after death to remove the soft tissue, the bones retain a high lipid content (Higgs et. al 2010). Particularly bones with a spongy (porous) structure have a high capacity to hold oil-rich marrow. Comparative data of various whale species suggests the skull, particularly the cranium and mandible bones are particularly oil rich. Along the vertebral column, the lipid content is reduced, particularly in the thoracic vertebrae (~10-25%), yet greatly increases from the lumbar to the caudal vertebrae (~40-55%). The chest area (scapula, sternum and ribs) show a mid-range lipid content (~15-30%), with vertically orientated ribs being more heavily soaked lower down (Turner Walker 2012, Higgs et. al 2010). Whale oil is largely composed of triglycerides (molecules of fatty acids attached to a glycerol molecule). In Arctic whales a higher proportion of unsaturated, versus saturated fatty acids make up the lipid. Unsaturated fatty acids (with double or triple carbon bonds causing chain kinks, preventing close packing (solidifying) of molecules), are more likely to be liquid (oil), versus solid (fat) at room temperature (Smith and March 2007). Objects Made From the Whaling Industry We all know that men set forth in sailing ships and risked their lives to harpoon whales on the open seas throughout the 1800s. And while Moby Dick and other tales have made whaling stories immortal, people today generally don't appreciate that the whalers were part of a well-organized industry. The ships that set out from ports in New England roamed as far as the Pacific in hunt of specific species of whales. Adventure may have been the draw for some whalers, but for the captains who owned whaling ships, and the investors which financed voyages, there was a considerable monetary payoff. The gigantic carcasses of whales were chopped and boiled down and turned into products such as the fine oil needed to lubricate increasing advanced machine tools. And beyond the oil derived from whales, even their bones, in an era before the invention of plastic, was used to make a wide variety of consumer goods. In short, whales were a valuable natural resource the same as wood, minerals, or petroleum we now pump from the ground. Oil From Whale’s Blubber Oil was the main product sought from whales, and it was used to lubricate machinery and to provide illumination by burning it in lamps. When a whale was killed, it was towed to the ship and its blubber, the thick insulating fat under its skin, would be peeled and cut from its carcass in a process known as “flensing.” The blubber was minced into chunks and boiled in large vats on board the whaling ship, producing oil. The oil taken from whale blubber was packaged in casks and transported back to the whaling ship’s home port (such as New Bedford, Massachusetts, the busiest American whaling port in the mid-1800s). From the ports it would be sold and transported across the country and would find its way into a huge variety of products. Whale oil, in addition to be used for lubrication and illumination, was also used to manufacture soaps, paint, and varnish. Whale oil was also utilized in some processes used to manufacture textiles and rope. Spermaceti, a Highly Regarded Oil A peculiar oil found in the head of the sperm whale, spermaceti, was highly prized. The oil was waxy, and was commonly used in making candles. In fact, candles made of spermaceti were considered the best in the world, producing a bright clear flame without an excess of smoke. Spermaceti was also used, distilled in liquid form, as an oil to fuel lamps. The main American whaling port, New Bedford, Massachusetts, was thus known as "The City That Lit the World." When John Adams was the ambassador to Great Britain before serving as president he recorded in his diary a conversation about spermaceti he had with the British Prime Minister William Pitt. Adams, keen to promote the New England whaling industry, was trying to convince the British to import spermaceti sold by American whalers, which the British could use to fuel street lamps. The British were not interested. In his diary, Adams wrote that he told Pitt, “the fat of the spermaceti whale gives the clearest and most beautiful flame of any substance that is known in nature, and we are surprised you prefer darkness, and consequent robberies, burglaries, and murders in your streets to receiving as a remittance our spermaceti oil.” Despite the failed sales pitch John Adams made in the late 1700s, the American whaling industry boomed in the early to mid-1800s. And spermaceti was a major component of that success. Spermaceti could be refined into a lubricant that was ideal for precision machinery. The machine tools that made the growth of industry possible in the United States were lubricated, and essentially made possible, by oil derived from spermaceti. Baleen, or "Whalebone" The bones and teeth of various species of whales were used in a number of products, many of them common implements in a 19th century household. Whales are said to have produced “the plastic of the 1800s.” The "bone" of the whale which was most commonly used wasn’t technically a bone, it was baleen, a hard material arrayed in large plates, like gigantic combs, in the mouths of some species of whales. The purpose of the baleen is to act as a sieve, catching tiny organisms in sea water, which the whale consumes as food. As baleen was tough yet flexible, it could be used in a number of practical applications. And it became commonly known as "whalebone." Perhaps the most common use of whalebone was in the manufacture of corsets, which fashionable ladies in the 1800s wore to compress their waistlines. One typical corset advertisement from the 1800s proudly proclaims, “Real Whalebone Only Used.” Whalebone was also used for collar stays, buggy whips, and toys. Its remarkable flexibility even caused it to be used as the springs in early typewriters. The comparison to plastic is apt. Think of common items which today might be made of plastic, and it's likely that similar items in the 1800s would have been made of whalebone. Baleen whales do not have teeth. But the teeth of other whales, such as the sperm whale, would be used as ivory in such products as chess pieces, piano keys, or the handles of walking sticks. Pieces of scrimshaw, or carved whale's teeth, would probably be the best remembered use of whale's teeth. However, the carved teeth were created to pass the time on whaling voyages and were never a mass production item. Their relative rarity, of course, is why genuine pieces of 19th century scrimshaw are considered to be valuable collectibles today. Reference: McNamara, Robert. "Objects Made From the Whaling Industry." ThoughtCo, Jul. 31, 2021, thoughtco.com/products-produced-from-whales-1774070. Whale bone was an important commodity, used in corsets, collar stays, buggy whips, and toys.Whale bone vertebrae. Advanced stage of calcification as indicated by deep pitting. Off white to grey.None.flagstaff hill, warrnambool, shipwrecked-coast, flagstaff-hill, flagstaff-hill-maritime-museum, maritime-museum, shipwreck-coast, flagstaff-hill-maritime-village, whales, whale bone, corsets, toys, whips -
 Flagstaff Hill Maritime Museum and Village
Flagstaff Hill Maritime Museum and VillageAnimal specimen - Whale bone, Undetermined
Prior to carrying out a detailed condition report of the cetacean skeletons, it is useful to have an understanding of the materials we are likely to encounter, in terms of structure and chemistry. This entry invites you to join in learning about the composition of whale bone and oil. Whale bone (Cetacean) bone is comprised of a composite structure of both an inorganic matrix of mainly hydroxylapatite (a calcium phosphate mineral), providing strength and rigidity, as well as an organic protein ‘scaffolding’ of mainly collagen, facilitating growth and repair (O’Connor 2008, CCI 2010). Collagen is also the structural protein component in cartilage between the whale vertebrae and attached to the fins of both the Killer Whale and the Dolphin. Relative proportions in the bone composition (affecting density), are linked with the feeding habits and mechanical stresses typically endured by bones of particular whale types. A Sperm Whale (Physeter macrocephalus Linnaeus, 1758) skeleton (toothed) thus has a higher mineral value (~67%) than a Fin Whale (Balaenoptera physalus Linnaeus, 1758) (baleen) (~60%) (Turner Walker 2012). The internal structure of bone can be divided into compact and cancellous bone. In whales, load-bearing structures such as mandibles and upper limb bones (e.g. humerus, sternum) are largely composed of compact bone (Turner Walker 2012). This consists of lamella concentrically deposited around the longitudinal axis and is permeated by fluid carrying channels (O’Connor 2008). Cancellous (spongy) bone, with a highly porous angular network of trabeculae, is less stiff and thus found in whale ribs and vertebrae (Turner Walker 2012). Whale oil Whales not only carry a thick layer of fat (blubber) in the soft tissue of their body for heat insulation and as a food store while they are alive, but also hold large oil (lipid) reserves in their porous bones. Following maceration of the whale skeleton after death to remove the soft tissue, the bones retain a high lipid content (Higgs et. al 2010). Particularly bones with a spongy (porous) structure have a high capacity to hold oil-rich marrow. Comparative data of various whale species suggests the skull, particularly the cranium and mandible bones are particularly oil rich. Along the vertebral column, the lipid content is reduced, particularly in the thoracic vertebrae (~10-25%), yet greatly increases from the lumbar to the caudal vertebrae (~40-55%). The chest area (scapula, sternum and ribs) show a mid-range lipid content (~15-30%), with vertically orientated ribs being more heavily soaked lower down (Turner Walker 2012, Higgs et. al 2010). Whale oil is largely composed of triglycerides (molecules of fatty acids attached to a glycerol molecule). In Arctic whales a higher proportion of unsaturated, versus saturated fatty acids make up the lipid. Unsaturated fatty acids (with double or triple carbon bonds causing chain kinks, preventing close packing (solidifying) of molecules), are more likely to be liquid (oil), versus solid (fat) at room temperature (Smith and March 2007). Objects Made From the Whaling Industry We all know that men set forth in sailing ships and risked their lives to harpoon whales on the open seas throughout the 1800s. And while Moby Dick and other tales have made whaling stories immortal, people today generally don't appreciate that the whalers were part of a well-organized industry. The ships that set out from ports in New England roamed as far as the Pacific in hunt of specific species of whales. Adventure may have been the draw for some whalers, but for the captains who owned whaling ships, and the investors which financed voyages, there was a considerable monetary payoff. The gigantic carcasses of whales were chopped and boiled down and turned into products such as the fine oil needed to lubricate increasing advanced machine tools. And beyond the oil derived from whales, even their bones, in an era before the invention of plastic, was used to make a wide variety of consumer goods. In short, whales were a valuable natural resource the same as wood, minerals, or petroleum we now pump from the ground. Oil From Whale’s Blubber Oil was the main product sought from whales, and it was used to lubricate machinery and to provide illumination by burning it in lamps. When a whale was killed, it was towed to the ship and its blubber, the thick insulating fat under its skin, would be peeled and cut from its carcass in a process known as “flensing.” The blubber was minced into chunks and boiled in large vats on board the whaling ship, producing oil. The oil taken from whale blubber was packaged in casks and transported back to the whaling ship’s home port (such as New Bedford, Massachusetts, the busiest American whaling port in the mid-1800s). From the ports it would be sold and transported across the country and would find its way into a huge variety of products. Whale oil, in addition to be used for lubrication and illumination, was also used to manufacture soaps, paint, and varnish. Whale oil was also utilized in some processes used to manufacture textiles and rope. Spermaceti, a Highly Regarded Oil A peculiar oil found in the head of the sperm whale, spermaceti, was highly prized. The oil was waxy, and was commonly used in making candles. In fact, candles made of spermaceti were considered the best in the world, producing a bright clear flame without an excess of smoke. Spermaceti was also used, distilled in liquid form, as an oil to fuel lamps. The main American whaling port, New Bedford, Massachusetts, was thus known as "The City That Lit the World." When John Adams was the ambassador to Great Britain before serving as president he recorded in his diary a conversation about spermaceti he had with the British Prime Minister William Pitt. Adams, keen to promote the New England whaling industry, was trying to convince the British to import spermaceti sold by American whalers, which the British could use to fuel street lamps. The British were not interested. In his diary, Adams wrote that he told Pitt, “the fat of the spermaceti whale gives the clearest and most beautiful flame of any substance that is known in nature, and we are surprised you prefer darkness, and consequent robberies, burglaries, and murders in your streets to receiving as a remittance our spermaceti oil.” Despite the failed sales pitch John Adams made in the late 1700s, the American whaling industry boomed in the early to mid-1800s. And spermaceti was a major component of that success. Spermaceti could be refined into a lubricant that was ideal for precision machinery. The machine tools that made the growth of industry possible in the United States were lubricated, and essentially made possible, by oil derived from spermaceti. Baleen, or "Whalebone" The bones and teeth of various species of whales were used in a number of products, many of them common implements in a 19th century household. Whales are said to have produced “the plastic of the 1800s.” The "bone" of the whale which was most commonly used wasn’t technically a bone, it was baleen, a hard material arrayed in large plates, like gigantic combs, in the mouths of some species of whales. The purpose of the baleen is to act as a sieve, catching tiny organisms in sea water, which the whale consumes as food. As baleen was tough yet flexible, it could be used in a number of practical applications. And it became commonly known as "whalebone." Perhaps the most common use of whalebone was in the manufacture of corsets, which fashionable ladies in the 1800s wore to compress their waistlines. One typical corset advertisement from the 1800s proudly proclaims, “Real Whalebone Only Used.” Whalebone was also used for collar stays, buggy whips, and toys. Its remarkable flexibility even caused it to be used as the springs in early typewriters. The comparison to plastic is apt. Think of common items which today might be made of plastic, and it's likely that similar items in the 1800s would have been made of whalebone. Baleen whales do not have teeth. But the teeth of other whales, such as the sperm whale, would be used as ivory in such products as chess pieces, piano keys, or the handles of walking sticks. Pieces of scrimshaw, or carved whale's teeth, would probably be the best remembered use of whale's teeth. However, the carved teeth were created to pass the time on whaling voyages and were never a mass production item. Their relative rarity, of course, is why genuine pieces of 19th century scrimshaw are considered to be valuable collectibles today. Reference: McNamara, Robert. "Objects Made From the Whaling Industry." ThoughtCo, Jul. 31, 2021, thoughtco.com/products-produced-from-whales-1774070.Whale bone was an important commodity, used in corsets, collar stays, buggy whips, and toys.Whale bone piece. Advanced stage of calcification as indicated by deep pitting. Off white to grey.None.flagstaff hill, warrnambool, shipwrecked-coast, flagstaff-hill, flagstaff-hill-maritime-museum, maritime-museum, shipwreck-coast, flagstaff-hill-maritime-village, whales, whale bone, corsets, toys, whips -
 Flagstaff Hill Maritime Museum and Village
Flagstaff Hill Maritime Museum and VillageAnimal specimen - Whale bone, Undetermined
Prior to carrying out a detailed condition report of the cetacean skeletons, it is useful to have an understanding of the materials we are likely to encounter, in terms of structure and chemistry. This entry invites you to join in learning about the composition of whale bone and oil. Whale bone (Cetacean) bone is comprised of a composite structure of both an inorganic matrix of mainly hydroxylapatite (a calcium phosphate mineral), providing strength and rigidity, as well as an organic protein ‘scaffolding’ of mainly collagen, facilitating growth and repair (O’Connor 2008, CCI 2010). Collagen is also the structural protein component in cartilage between the whale vertebrae and attached to the fins of both the Killer Whale and the Dolphin. Relative proportions in the bone composition (affecting density), are linked with the feeding habits and mechanical stresses typically endured by bones of particular whale types. A Sperm Whale (Physeter macrocephalus Linnaeus, 1758) skeleton (toothed) thus has a higher mineral value (~67%) than a Fin Whale (Balaenoptera physalus Linnaeus, 1758) (baleen) (~60%) (Turner Walker 2012). The internal structure of bone can be divided into compact and cancellous bone. In whales, load-bearing structures such as mandibles and upper limb bones (e.g. humerus, sternum) are largely composed of compact bone (Turner Walker 2012). This consists of lamella concentrically deposited around the longitudinal axis and is permeated by fluid carrying channels (O’Connor 2008). Cancellous (spongy) bone, with a highly porous angular network of trabeculae, is less stiff and thus found in whale ribs and vertebrae (Turner Walker 2012). Whale oil Whales not only carry a thick layer of fat (blubber) in the soft tissue of their body for heat insulation and as a food store while they are alive, but also hold large oil (lipid) reserves in their porous bones. Following maceration of the whale skeleton after death to remove the soft tissue, the bones retain a high lipid content (Higgs et. al 2010). Particularly bones with a spongy (porous) structure have a high capacity to hold oil-rich marrow. Comparative data of various whale species suggests the skull, particularly the cranium and mandible bones are particularly oil rich. Along the vertebral column, the lipid content is reduced, particularly in the thoracic vertebrae (~10-25%), yet greatly increases from the lumbar to the caudal vertebrae (~40-55%). The chest area (scapula, sternum and ribs) show a mid-range lipid content (~15-30%), with vertically orientated ribs being more heavily soaked lower down (Turner Walker 2012, Higgs et. al 2010). Whale oil is largely composed of triglycerides (molecules of fatty acids attached to a glycerol molecule). In Arctic whales a higher proportion of unsaturated, versus saturated fatty acids make up the lipid. Unsaturated fatty acids (with double or triple carbon bonds causing chain kinks, preventing close packing (solidifying) of molecules), are more likely to be liquid (oil), versus solid (fat) at room temperature (Smith and March 2007). Objects Made From the Whaling Industry We all know that men set forth in sailing ships and risked their lives to harpoon whales on the open seas throughout the 1800s. And while Moby Dick and other tales have made whaling stories immortal, people today generally don't appreciate that the whalers were part of a well-organized industry. The ships that set out from ports in New England roamed as far as the Pacific in hunt of specific species of whales. Adventure may have been the draw for some whalers, but for the captains who owned whaling ships, and the investors which financed voyages, there was a considerable monetary payoff. The gigantic carcasses of whales were chopped and boiled down and turned into products such as the fine oil needed to lubricate increasing advanced machine tools. And beyond the oil derived from whales, even their bones, in an era before the invention of plastic, was used to make a wide variety of consumer goods. In short, whales were a valuable natural resource the same as wood, minerals, or petroleum we now pump from the ground. Oil From Whale’s Blubber Oil was the main product sought from whales, and it was used to lubricate machinery and to provide illumination by burning it in lamps. When a whale was killed, it was towed to the ship and its blubber, the thick insulating fat under its skin, would be peeled and cut from its carcass in a process known as “flensing.” The blubber was minced into chunks and boiled in large vats on board the whaling ship, producing oil. The oil taken from whale blubber was packaged in casks and transported back to the whaling ship’s home port (such as New Bedford, Massachusetts, the busiest American whaling port in the mid-1800s). From the ports it would be sold and transported across the country and would find its way into a huge variety of products. Whale oil, in addition to be used for lubrication and illumination, was also used to manufacture soaps, paint, and varnish. Whale oil was also utilized in some processes used to manufacture textiles and rope. Spermaceti, a Highly Regarded Oil A peculiar oil found in the head of the sperm whale, spermaceti, was highly prized. The oil was waxy, and was commonly used in making candles. In fact, candles made of spermaceti were considered the best in the world, producing a bright clear flame without an excess of smoke. Spermaceti was also used, distilled in liquid form, as an oil to fuel lamps. The main American whaling port, New Bedford, Massachusetts, was thus known as "The City That Lit the World." When John Adams was the ambassador to Great Britain before serving as president he recorded in his diary a conversation about spermaceti he had with the British Prime Minister William Pitt. Adams, keen to promote the New England whaling industry, was trying to convince the British to import spermaceti sold by American whalers, which the British could use to fuel street lamps. The British were not interested. In his diary, Adams wrote that he told Pitt, “the fat of the spermaceti whale gives the clearest and most beautiful flame of any substance that is known in nature, and we are surprised you prefer darkness, and consequent robberies, burglaries, and murders in your streets to receiving as a remittance our spermaceti oil.” Despite the failed sales pitch John Adams made in the late 1700s, the American whaling industry boomed in the early to mid-1800s. And spermaceti was a major component of that success. Spermaceti could be refined into a lubricant that was ideal for precision machinery. The machine tools that made the growth of industry possible in the United States were lubricated, and essentially made possible, by oil derived from spermaceti. Baleen, or "Whalebone" The bones and teeth of various species of whales were used in a number of products, many of them common implements in a 19th century household. Whales are said to have produced “the plastic of the 1800s.” The "bone" of the whale which was most commonly used wasn’t technically a bone, it was baleen, a hard material arrayed in large plates, like gigantic combs, in the mouths of some species of whales. The purpose of the baleen is to act as a sieve, catching tiny organisms in sea water, which the whale consumes as food. As baleen was tough yet flexible, it could be used in a number of practical applications. And it became commonly known as "whalebone." Perhaps the most common use of whalebone was in the manufacture of corsets, which fashionable ladies in the 1800s wore to compress their waistlines. One typical corset advertisement from the 1800s proudly proclaims, “Real Whalebone Only Used.” Whalebone was also used for collar stays, buggy whips, and toys. Its remarkable flexibility even caused it to be used as the springs in early typewriters. The comparison to plastic is apt. Think of common items which today might be made of plastic, and it's likely that similar items in the 1800s would have been made of whalebone. Baleen whales do not have teeth. But the teeth of other whales, such as the sperm whale, would be used as ivory in such products as chess pieces, piano keys, or the handles of walking sticks. Pieces of scrimshaw, or carved whale's teeth, would probably be the best remembered use of whale's teeth. However, the carved teeth were created to pass the time on whaling voyages and were never a mass production item. Their relative rarity, of course, is why genuine pieces of 19th century scrimshaw are considered to be valuable collectibles today. Reference: McNamara, Robert. "Objects Made From the Whaling Industry." ThoughtCo, Jul. 31, 2021, thoughtco.com/products-produced-from-whales-1774070.Whale bone was an important commodity, used in corsets, collar stays, buggy whips, and toys.Whale bone piece. Advanced stage of calcification as indicated by deep pitting. Off white to grey.None.flagstaff hill, warrnambool, shipwrecked-coast, flagstaff-hill, flagstaff-hill-maritime-museum, maritime-museum, shipwreck-coast, flagstaff-hill-maritime-village, whales, whale bone, corsets, toys, whips -
 Flagstaff Hill Maritime Museum and Village
Flagstaff Hill Maritime Museum and VillageAnimal specimen - Whale Rib Bone, Undetermined
Prior to carrying out a detailed condition report of the cetacean skeletons, it is useful to have an understanding of the materials we are likely to encounter, in terms of structure and chemistry. This entry invites you to join in learning about the composition of whale bone and oil. Whale bone (Cetacean) bone is comprised of a composite structure of both an inorganic matrix of mainly hydroxylapatite (a calcium phosphate mineral), providing strength and rigidity, as well as an organic protein ‘scaffolding’ of mainly collagen, facilitating growth and repair (O’Connor 2008, CCI 2010). Collagen is also the structural protein component in cartilage between the whale vertebrae and attached to the fins of both the Killer Whale and the Dolphin. Relative proportions in the bone composition (affecting density), are linked with the feeding habits and mechanical stresses typically endured by bones of particular whale types. A Sperm Whale (Physeter macrocephalus Linnaeus, 1758) skeleton (toothed) thus has a higher mineral value (~67%) than a Fin Whale (Balaenoptera physalus Linnaeus, 1758) (baleen) (~60%) (Turner Walker 2012). The internal structure of bone can be divided into compact and cancellous bone. In whales, load-bearing structures such as mandibles and upper limb bones (e.g. humerus, sternum) are largely composed of compact bone (Turner Walker 2012). This consists of lamella concentrically deposited around the longitudinal axis and is permeated by fluid carrying channels (O’Connor 2008). Cancellous (spongy) bone, with a highly porous angular network of trabeculae, is less stiff and thus found in whale ribs and vertebrae (Turner Walker 2012). Whale oil Whales not only carry a thick layer of fat (blubber) in the soft tissue of their body for heat insulation and as a food store while they are alive, but also hold large oil (lipid) reserves in their porous bones. Following maceration of the whale skeleton after death to remove the soft tissue, the bones retain a high lipid content (Higgs et. al 2010). Particularly bones with a spongy (porous) structure have a high capacity to hold oil-rich marrow. Comparative data of various whale species suggests the skull, particularly the cranium and mandible bones are particularly oil rich. Along the vertebral column, the lipid content is reduced, particularly in the thoracic vertebrae (~10-25%), yet greatly increases from the lumbar to the caudal vertebrae (~40-55%). The chest area (scapula, sternum and ribs) show a mid-range lipid content (~15-30%), with vertically orientated ribs being more heavily soaked lower down (Turner Walker 2012, Higgs et. al 2010). Whale oil is largely composed of triglycerides (molecules of fatty acids attached to a glycerol molecule). In Arctic whales a higher proportion of unsaturated, versus saturated fatty acids make up the lipid. Unsaturated fatty acids (with double or triple carbon bonds causing chain kinks, preventing close packing (solidifying) of molecules), are more likely to be liquid (oil), versus solid (fat) at room temperature (Smith and March 2007). Objects Made From the Whaling Industry We all know that men set forth in sailing ships and risked their lives to harpoon whales on the open seas throughout the 1800s. And while Moby Dick and other tales have made whaling stories immortal, people today generally don't appreciate that the whalers were part of a well-organized industry. The ships that set out from ports in New England roamed as far as the Pacific in hunt of specific species of whales. Adventure may have been the draw for some whalers, but for the captains who owned whaling ships, and the investors which financed voyages, there was a considerable monetary payoff. The gigantic carcasses of whales were chopped and boiled down and turned into products such as the fine oil needed to lubricate increasing advanced machine tools. And beyond the oil derived from whales, even their bones, in an era before the invention of plastic, was used to make a wide variety of consumer goods. In short, whales were a valuable natural resource the same as wood, minerals, or petroleum we now pump from the ground. Oil From Whale’s Blubber Oil was the main product sought from whales, and it was used to lubricate machinery and to provide illumination by burning it in lamps. When a whale was killed, it was towed to the ship and its blubber, the thick insulating fat under its skin, would be peeled and cut from its carcass in a process known as “flensing.” The blubber was minced into chunks and boiled in large vats on board the whaling ship, producing oil. The oil taken from whale blubber was packaged in casks and transported back to the whaling ship’s home port (such as New Bedford, Massachusetts, the busiest American whaling port in the mid-1800s). From the ports it would be sold and transported across the country and would find its way into a huge variety of products. Whale oil, in addition to be used for lubrication and illumination, was also used to manufacture soaps, paint, and varnish. Whale oil was also utilized in some processes used to manufacture textiles and rope. Spermaceti, a Highly Regarded Oil A peculiar oil found in the head of the sperm whale, spermaceti, was highly prized. The oil was waxy, and was commonly used in making candles. In fact, candles made of spermaceti were considered the best in the world, producing a bright clear flame without an excess of smoke. Spermaceti was also used, distilled in liquid form, as an oil to fuel lamps. The main American whaling port, New Bedford, Massachusetts, was thus known as "The City That Lit the World." When John Adams was the ambassador to Great Britain before serving as president he recorded in his diary a conversation about spermaceti he had with the British Prime Minister William Pitt. Adams, keen to promote the New England whaling industry, was trying to convince the British to import spermaceti sold by American whalers, which the British could use to fuel street lamps. The British were not interested. In his diary, Adams wrote that he told Pitt, “the fat of the spermaceti whale gives the clearest and most beautiful flame of any substance that is known in nature, and we are surprised you prefer darkness, and consequent robberies, burglaries, and murders in your streets to receiving as a remittance our spermaceti oil.” Despite the failed sales pitch John Adams made in the late 1700s, the American whaling industry boomed in the early to mid-1800s. And spermaceti was a major component of that success. Spermaceti could be refined into a lubricant that was ideal for precision machinery. The machine tools that made the growth of industry possible in the United States were lubricated, and essentially made possible, by oil derived from spermaceti. Baleen, or "Whalebone" The bones and teeth of various species of whales were used in a number of products, many of them common implements in a 19th century household. Whales are said to have produced “the plastic of the 1800s.” The "bone" of the whale which was most commonly used wasn’t technically a bone, it was baleen, a hard material arrayed in large plates, like gigantic combs, in the mouths of some species of whales. The purpose of the baleen is to act as a sieve, catching tiny organisms in sea water, which the whale consumes as food. As baleen was tough yet flexible, it could be used in a number of practical applications. And it became commonly known as "whalebone." Perhaps the most common use of whalebone was in the manufacture of corsets, which fashionable ladies in the 1800s wore to compress their waistlines. One typical corset advertisement from the 1800s proudly proclaims, “Real Whalebone Only Used.” Whalebone was also used for collar stays, buggy whips, and toys. Its remarkable flexibility even caused it to be used as the springs in early typewriters. The comparison to plastic is apt. Think of common items which today might be made of plastic, and it's likely that similar items in the 1800s would have been made of whalebone. Baleen whales do not have teeth. But the teeth of other whales, such as the sperm whale, would be used as ivory in such products as chess pieces, piano keys, or the handles of walking sticks. Pieces of scrimshaw, or carved whale's teeth, would probably be the best remembered use of whale's teeth. However, the carved teeth were created to pass the time on whaling voyages and were never a mass production item. Their relative rarity, of course, is why genuine pieces of 19th century scrimshaw are considered to be valuable collectibles today. Reference: McNamara, Robert. "Objects Made From the Whaling Industry." ThoughtCo, Jul. 31, 2021, thoughtco.com/products-produced-from-whales-1774070.Whale bone during the 17th, 18th, 19th and early 20th centuries was an important industry providing an important commodity. Whales from these times provided everything from lighting & machine oils to using the animal's bones for use in corsets, collar stays, buggy whips, and many other everyday items then in use.Whale rib bone with advanced stage of calcification as indicated by brittleness. None.warrnambool, flagstaff-hill, flagstaff-hill-maritime-museum, maritime-museum, shipwreck-coast, flagstaff-hill-maritime-village, whale bones, whale skeleton, whales, whale bone, corsets, toys, whips, whaleling industry, maritime fishing, whalebone -
 Flagstaff Hill Maritime Museum and Village
Flagstaff Hill Maritime Museum and VillageAnimal specimen - Whale bone, Undetermined
Prior to carrying out a detailed condition report of the cetacean skeletons, it is useful to have an understanding of the materials we are likely to encounter, in terms of structure and chemistry. This entry invites you to join in learning about the composition of whale bone and oil. Whale bone (Cetacean) bone is comprised of a composite structure of both an inorganic matrix of mainly hydroxylapatite (a calcium phosphate mineral), providing strength and rigidity, as well as an organic protein ‘scaffolding’ of mainly collagen, facilitating growth and repair (O’Connor 2008, CCI 2010). Collagen is also the structural protein component in cartilage between the whale vertebrae and attached to the fins of both the Killer Whale and the Dolphin. Relative proportions in the bone composition (affecting density), are linked with the feeding habits and mechanical stresses typically endured by bones of particular whale types. A Sperm Whale (Physeter macrocephalus Linnaeus, 1758) skeleton (toothed) thus has a higher mineral value (~67%) than a Fin Whale (Balaenoptera physalus Linnaeus, 1758) (baleen) (~60%) (Turner Walker 2012). The internal structure of bone can be divided into compact and cancellous bone. In whales, load-bearing structures such as mandibles and upper limb bones (e.g. humerus, sternum) are largely composed of compact bone (Turner Walker 2012). This consists of lamella concentrically deposited around the longitudinal axis and is permeated by fluid carrying channels (O’Connor 2008). Cancellous (spongy) bone, with a highly porous angular network of trabeculae, is less stiff and thus found in whale ribs and vertebrae (Turner Walker 2012). Whale oil Whales not only carry a thick layer of fat (blubber) in the soft tissue of their body for heat insulation and as a food store while they are alive, but also hold large oil (lipid) reserves in their porous bones. Following maceration of the whale skeleton after death to remove the soft tissue, the bones retain a high lipid content (Higgs et. al 2010). Particularly bones with a spongy (porous) structure have a high capacity to hold oil-rich marrow. Comparative data of various whale species suggests the skull, particularly the cranium and mandible bones are particularly oil rich. Along the vertebral column, the lipid content is reduced, particularly in the thoracic vertebrae (~10-25%), yet greatly increases from the lumbar to the caudal vertebrae (~40-55%). The chest area (scapula, sternum and ribs) show a mid-range lipid content (~15-30%), with vertically orientated ribs being more heavily soaked lower down (Turner Walker 2012, Higgs et. al 2010). Whale oil is largely composed of triglycerides (molecules of fatty acids attached to a glycerol molecule). In Arctic whales a higher proportion of unsaturated, versus saturated fatty acids make up the lipid. Unsaturated fatty acids (with double or triple carbon bonds causing chain kinks, preventing close packing (solidifying) of molecules), are more likely to be liquid (oil), versus solid (fat) at room temperature (Smith and March 2007). Objects Made From the Whaling Industry We all know that men set forth in sailing ships and risked their lives to harpoon whales on the open seas throughout the 1800s. And while Moby Dick and other tales have made whaling stories immortal, people today generally don't appreciate that the whalers were part of a well-organized industry. The ships that set out from ports in New England roamed as far as the Pacific in hunt of specific species of whales. Adventure may have been the draw for some whalers, but for the captains who owned whaling ships, and the investors which financed voyages, there was a considerable monetary payoff. The gigantic carcasses of whales were chopped and boiled down and turned into products such as the fine oil needed to lubricate increasing advanced machine tools. And beyond the oil derived from whales, even their bones, in an era before the invention of plastic, was used to make a wide variety of consumer goods. In short, whales were a valuable natural resource the same as wood, minerals, or petroleum we now pump from the ground. Oil From Whale’s Blubber Oil was the main product sought from whales, and it was used to lubricate machinery and to provide illumination by burning it in lamps. When a whale was killed, it was towed to the ship and its blubber, the thick insulating fat under its skin, would be peeled and cut from its carcass in a process known as “flensing.” The blubber was minced into chunks and boiled in large vats on board the whaling ship, producing oil. The oil taken from whale blubber was packaged in casks and transported back to the whaling ship’s home port (such as New Bedford, Massachusetts, the busiest American whaling port in the mid-1800s). From the ports it would be sold and transported across the country and would find its way into a huge variety of products. Whale oil, in addition to be used for lubrication and illumination, was also used to manufacture soaps, paint, and varnish. Whale oil was also utilized in some processes used to manufacture textiles and rope. Spermaceti, a Highly Regarded Oil A peculiar oil found in the head of the sperm whale, spermaceti, was highly prized. The oil was waxy, and was commonly used in making candles. In fact, candles made of spermaceti were considered the best in the world, producing a bright clear flame without an excess of smoke. Spermaceti was also used, distilled in liquid form, as an oil to fuel lamps. The main American whaling port, New Bedford, Massachusetts, was thus known as "The City That Lit the World." When John Adams was the ambassador to Great Britain before serving as president he recorded in his diary a conversation about spermaceti he had with the British Prime Minister William Pitt. Adams, keen to promote the New England whaling industry, was trying to convince the British to import spermaceti sold by American whalers, which the British could use to fuel street lamps. The British were not interested. In his diary, Adams wrote that he told Pitt, “the fat of the spermaceti whale gives the clearest and most beautiful flame of any substance that is known in nature, and we are surprised you prefer darkness, and consequent robberies, burglaries, and murders in your streets to receiving as a remittance our spermaceti oil.” Despite the failed sales pitch John Adams made in the late 1700s, the American whaling industry boomed in the early to mid-1800s. And spermaceti was a major component of that success. Spermaceti could be refined into a lubricant that was ideal for precision machinery. The machine tools that made the growth of industry possible in the United States were lubricated, and essentially made possible, by oil derived from spermaceti. Baleen, or "Whalebone" The bones and teeth of various species of whales were used in a number of products, many of them common implements in a 19th century household. Whales are said to have produced “the plastic of the 1800s.” The "bone" of the whale which was most commonly used wasn’t technically a bone, it was baleen, a hard material arrayed in large plates, like gigantic combs, in the mouths of some species of whales. The purpose of the baleen is to act as a sieve, catching tiny organisms in sea water, which the whale consumes as food. As baleen was tough yet flexible, it could be used in a number of practical applications. And it became commonly known as "whalebone." Perhaps the most common use of whalebone was in the manufacture of corsets, which fashionable ladies in the 1800s wore to compress their waistlines. One typical corset advertisement from the 1800s proudly proclaims, “Real Whalebone Only Used.” Whalebone was also used for collar stays, buggy whips, and toys. Its remarkable flexibility even caused it to be used as the springs in early typewriters. The comparison to plastic is apt. Think of common items which today might be made of plastic, and it's likely that similar items in the 1800s would have been made of whalebone. Baleen whales do not have teeth. But the teeth of other whales, such as the sperm whale, would be used as ivory in such products as chess pieces, piano keys, or the handles of walking sticks. Pieces of scrimshaw, or carved whale's teeth, would probably be the best remembered use of whale's teeth. However, the carved teeth were created to pass the time on whaling voyages and were never a mass production item. Their relative rarity, of course, is why genuine pieces of 19th century scrimshaw are considered to be valuable collectibles today. Reference: McNamara, Robert. "Objects Made From the Whaling Industry." ThoughtCo, Jul. 31, 2021, thoughtco.com/products-produced-from-whales-1774070.Whale bone was an important commodity, used in corsets, collar stays, buggy whips, and toys.Whale bone vertebrae. Advanced stage of calcification as indicated by deep pitting. Off white to grey.Noneflagstaff hill, warrnambool, shipwrecked-coast, flagstaff-hill, flagstaff-hill-maritime-museum, maritime-museum, shipwreck-coast, flagstaff-hill-maritime-village, whales, whale bone, corsets, toys, whips, whalebone -
 Flagstaff Hill Maritime Museum and Village
Flagstaff Hill Maritime Museum and VillageAnimal specimen - Whale Vertebrae, Undetermined
Prior to carrying out a detailed condition report of the cetacean skeletons, it is useful to have an understanding of the materials we are likely to encounter, in terms of structure and chemistry. This entry invites you to join in learning about the composition of whale bone and oil. Whale bone (Cetacean) bone is comprised of a composite structure of both an inorganic matrix of mainly hydroxylapatite (a calcium phosphate mineral), providing strength and rigidity, as well as an organic protein ‘scaffolding’ of mainly collagen, facilitating growth and repair (O’Connor 2008, CCI 2010). Collagen is also the structural protein component in cartilage between the whale vertebrae and attached to the fins of both the Killer Whale and the Dolphin. Relative proportions in the bone composition (affecting density), are linked with the feeding habits and mechanical stresses typically endured by bones of particular whale types. A Sperm Whale (Physeter macrocephalus Linnaeus, 1758) skeleton (toothed) thus has a higher mineral value (~67%) than a Fin Whale (Balaenoptera physalus Linnaeus, 1758) (baleen) (~60%) (Turner Walker 2012). The internal structure of bone can be divided into compact and cancellous bone. In whales, load-bearing structures such as mandibles and upper limb bones (e.g. humerus, sternum) are largely composed of compact bone (Turner Walker 2012). This consists of lamella concentrically deposited around the longitudinal axis and is permeated by fluid carrying channels (O’Connor 2008). Cancellous (spongy) bone, with a highly porous angular network of trabeculae, is less stiff and thus found in whale ribs and vertebrae (Turner Walker 2012). Whale oil Whales not only carry a thick layer of fat (blubber) in the soft tissue of their body for heat insulation and as a food store while they are alive, but also hold large oil (lipid) reserves in their porous bones. Following maceration of the whale skeleton after death to remove the soft tissue, the bones retain a high lipid content (Higgs et. al 2010). Particularly bones with a spongy (porous) structure have a high capacity to hold oil-rich marrow. Comparative data of various whale species suggests the skull, particularly the cranium and mandible bones are particularly oil rich. Along the vertebral column, the lipid content is reduced, particularly in the thoracic vertebrae (~10-25%), yet greatly increases from the lumbar to the caudal vertebrae (~40-55%). The chest area (scapula, sternum and ribs) show a mid-range lipid content (~15-30%), with vertically orientated ribs being more heavily soaked lower down (Turner Walker 2012, Higgs et. al 2010). Whale oil is largely composed of triglycerides (molecules of fatty acids attached to a glycerol molecule). In Arctic whales a higher proportion of unsaturated, versus saturated fatty acids make up the lipid. Unsaturated fatty acids (with double or triple carbon bonds causing chain kinks, preventing close packing (solidifying) of molecules), are more likely to be liquid (oil), versus solid (fat) at room temperature (Smith and March 2007). Objects Made From the Whaling Industry We all know that men set forth in sailing ships and risked their lives to harpoon whales on the open seas throughout the 1800s. And while Moby Dick and other tales have made whaling stories immortal, people today generally don't appreciate that the whalers were part of a well-organized industry. The ships that set out from ports in New England roamed as far as the Pacific in hunt of specific species of whales. Adventure may have been the draw for some whalers, but for the captains who owned whaling ships, and the investors which financed voyages, there was a considerable monetary payoff. The gigantic carcasses of whales were chopped and boiled down and turned into products such as the fine oil needed to lubricate increasing advanced machine tools. And beyond the oil derived from whales, even their bones, in an era before the invention of plastic, was used to make a wide variety of consumer goods. In short, whales were a valuable natural resource the same as wood, minerals, or petroleum we now pump from the ground. Oil From Whale’s Blubber Oil was the main product sought from whales, and it was used to lubricate machinery and to provide illumination by burning it in lamps. When a whale was killed, it was towed to the ship and its blubber, the thick insulating fat under its skin, would be peeled and cut from its carcass in a process known as “flensing.” The blubber was minced into chunks and boiled in large vats on board the whaling ship, producing oil. The oil taken from whale blubber was packaged in casks and transported back to the whaling ship’s home port (such as New Bedford, Massachusetts, the busiest American whaling port in the mid-1800s). From the ports it would be sold and transported across the country and would find its way into a huge variety of products. Whale oil, in addition to be used for lubrication and illumination, was also used to manufacture soaps, paint, and varnish. Whale oil was also utilized in some processes used to manufacture textiles and rope. Spermaceti, a Highly Regarded Oil A peculiar oil found in the head of the sperm whale, spermaceti, was highly prized. The oil was waxy, and was commonly used in making candles. In fact, candles made of spermaceti were considered the best in the world, producing a bright clear flame without an excess of smoke. Spermaceti was also used, distilled in liquid form, as an oil to fuel lamps. The main American whaling port, New Bedford, Massachusetts, was thus known as "The City That Lit the World." When John Adams was the ambassador to Great Britain before serving as president he recorded in his diary a conversation about spermaceti he had with the British Prime Minister William Pitt. Adams, keen to promote the New England whaling industry, was trying to convince the British to import spermaceti sold by American whalers, which the British could use to fuel street lamps. The British were not interested. In his diary, Adams wrote that he told Pitt, “the fat of the spermaceti whale gives the clearest and most beautiful flame of any substance that is known in nature, and we are surprised you prefer darkness, and consequent robberies, burglaries, and murders in your streets to receiving as a remittance our spermaceti oil.” Despite the failed sales pitch John Adams made in the late 1700s, the American whaling industry boomed in the early to mid-1800s. And spermaceti was a major component of that success. Spermaceti could be refined into a lubricant that was ideal for precision machinery. The machine tools that made the growth of industry possible in the United States were lubricated, and essentially made possible, by oil derived from spermaceti. Whalebone The bones and teeth of various species of whales were used in a number of products, many of them common implements in a 19th century household. Whales are said to have produced “the plastic of the 1800s.” The bone of the whale which was most commonly used wasn’t technically a bone, it was baleen, a hard material arrayed in large plates, like gigantic combs, in the mouths of some species of whales. The purpose of the baleen is to act as a sieve, catching tiny organisms in sea water, which the whale consumes as food. As baleen was tough yet flexible, it could be used in a number of practical applications. And it became commonly known as whalebone. Perhaps the most common use of whalebone was in the manufacture of corsets, which fashionable ladies in the 1800s wore to compress their waistlines. One typical corset advertisement from the 1800s proudly proclaims, “Real Whalebone Only Used.” Whalebone was also used for collar stays, buggy whips, and toys. Its remarkable flexibility even caused it to be used as the springs in early typewriters. The comparison to plastic is apt. Think of common items which today might be made of plastic, and it's likely that similar items in the 1800s would have been made of whalebone. Baleen whales do not have teeth. But the teeth of other whales, such as the sperm whale, would be used as ivory in such products as chess pieces, piano keys, or the handles of walking sticks. Pieces of scrimshaw, or carved whale's teeth, would probably be the best remembered use of whale's teeth. However, the carved teeth were created to pass the time on whaling voyages and were never a mass production item. Their relative rarity, of course, is why genuine pieces of 19th century scrimshaw are considered to be valuable collectibles today. Reference: McNamara, Robert. "Objects Made From the Whaling Industry." ThoughtCo, Jul. 31, 2021, thoughtco.com/products-produced-from-whales-1774070.Whale bone during the 17th, 18th, 19th and early 20th centuries was an important industry providing an important commodity. Whales from these times provided everything from lighting & machine oils to using the animal's bones for use in corsets, collar stays, buggy whips, and many other everyday items then in use.Whale bone Vertebrae with advanced stage of calcification as indicated by deep pitting. Off white to grey.None.warrnambool, flagstaff-hill, flagstaff-hill-maritime-museum, maritime-museum, shipwreck-coast, flagstaff-hill-maritime-village, whale bones, whale skeleton, whales, whale bone, corsets, toys, whips, whaleling industry, maritime fishing, whalebone -
 Flagstaff Hill Maritime Museum and Village
Flagstaff Hill Maritime Museum and VillageAnimal specimen - Whale Jaw Bone, Undetermined
Prior to carrying out a detailed condition report of the cetacean skeletons, it is useful to have an understanding of the materials we are likely to encounter, in terms of structure and chemistry. This entry invites you to join in learning about the composition of whale bone and oil. Whale bone (Cetacean) bone is comprised of a composite structure of both an inorganic matrix of mainly hydroxylapatite (a calcium phosphate mineral), providing strength and rigidity, as well as an organic protein ‘scaffolding’ of mainly collagen, facilitating growth and repair (O’Connor 2008, CCI 2010). Collagen is also the structural protein component in cartilage between the whale vertebrae and attached to the fins of both the Killer Whale and the Dolphin. Relative proportions in the bone composition (affecting density), are linked with the feeding habits and mechanical stresses typically endured by bones of particular whale types. A Sperm Whale (Physeter macrocephalus Linnaeus, 1758) skeleton (toothed) thus has a higher mineral value (~67%) than a Fin Whale (Balaenoptera physalus Linnaeus, 1758) (baleen) (~60%) (Turner Walker 2012). The internal structure of bone can be divided into compact and cancellous bone. In whales, load-bearing structures such as mandibles and upper limb bones (e.g. humerus, sternum) are largely composed of compact bone (Turner Walker 2012). This consists of lamella concentrically deposited around the longitudinal axis and is permeated by fluid carrying channels (O’Connor 2008). Cancellous (spongy) bone, with a highly porous angular network of trabeculae, is less stiff and thus found in whale ribs and vertebrae (Turner Walker 2012). Whale oil Whales not only carry a thick layer of fat (blubber) in the soft tissue of their body for heat insulation and as a food store while they are alive, but also hold large oil (lipid) reserves in their porous bones. Following maceration of the whale skeleton after death to remove the soft tissue, the bones retain a high lipid content (Higgs et. al 2010). Particularly bones with a spongy (porous) structure have a high capacity to hold oil-rich marrow. Comparative data of various whale species suggests the skull, particularly the cranium and mandible bones are particularly oil rich. Along the vertebral column, the lipid content is reduced, particularly in the thoracic vertebrae (~10-25%), yet greatly increases from the lumbar to the caudal vertebrae (~40-55%). The chest area (scapula, sternum and ribs) show a mid-range lipid content (~15-30%), with vertically orientated ribs being more heavily soaked lower down (Turner Walker 2012, Higgs et. al 2010). Whale oil is largely composed of triglycerides (molecules of fatty acids attached to a glycerol molecule). In Arctic whales a higher proportion of unsaturated, versus saturated fatty acids make up the lipid. Unsaturated fatty acids (with double or triple carbon bonds causing chain kinks, preventing close packing (solidifying) of molecules), are more likely to be liquid (oil), versus solid (fat) at room temperature (Smith and March 2007). Objects Made From the Whaling Industry We all know that men set forth in sailing ships and risked their lives to harpoon whales on the open seas throughout the 1800s. And while Moby Dick and other tales have made whaling stories immortal, people today generally don't appreciate that the whalers were part of a well-organized industry. The ships that set out from ports in New England roamed as far as the Pacific in hunt of specific species of whales. Adventure may have been the draw for some whalers, but for the captains who owned whaling ships, and the investors which financed voyages, there was a considerable monetary payoff. The gigantic carcasses of whales were chopped and boiled down and turned into products such as the fine oil needed to lubricate increasing advanced machine tools. And beyond the oil derived from whales, even their bones, in an era before the invention of plastic, was used to make a wide variety of consumer goods. In short, whales were a valuable natural resource the same as wood, minerals, or petroleum we now pump from the ground. Oil From Whale’s Blubber Oil was the main product sought from whales, and it was used to lubricate machinery and to provide illumination by burning it in lamps. When a whale was killed, it was towed to the ship and its blubber, the thick insulating fat under its skin, would be peeled and cut from its carcass in a process known as “flensing.” The blubber was minced into chunks and boiled in large vats on board the whaling ship, producing oil. The oil taken from whale blubber was packaged in casks and transported back to the whaling ship’s home port (such as New Bedford, Massachusetts, the busiest American whaling port in the mid-1800s). From the ports it would be sold and transported across the country and would find its way into a huge variety of products. Whale oil, in addition to be used for lubrication and illumination, was also used to manufacture soaps, paint, and varnish. Whale oil was also utilized in some processes used to manufacture textiles and rope. Spermaceti, a Highly Regarded Oil A peculiar oil found in the head of the sperm whale, spermaceti, was highly prized. The oil was waxy, and was commonly used in making candles. In fact, candles made of spermaceti were considered the best in the world, producing a bright clear flame without an excess of smoke. Spermaceti was also used, distilled in liquid form, as an oil to fuel lamps. The main American whaling port, New Bedford, Massachusetts, was thus known as "The City That Lit the World." When John Adams was the ambassador to Great Britain before serving as president he recorded in his diary a conversation about spermaceti he had with the British Prime Minister William Pitt. Adams, keen to promote the New England whaling industry, was trying to convince the British to import spermaceti sold by American whalers, which the British could use to fuel street lamps. The British were not interested. In his diary, Adams wrote that he told Pitt, “the fat of the spermaceti whale gives the clearest and most beautiful flame of any substance that is known in nature, and we are surprised you prefer darkness, and consequent robberies, burglaries, and murders in your streets to receiving as a remittance our spermaceti oil.” Despite the failed sales pitch John Adams made in the late 1700s, the American whaling industry boomed in the early to mid-1800s. And spermaceti was a major component of that success. Spermaceti could be refined into a lubricant that was ideal for precision machinery. The machine tools that made the growth of industry possible in the United States were lubricated, and essentially made possible, by oil derived from spermaceti. Baleen, or "Whalebone" The bones and teeth of various species of whales were used in a number of products, many of them common implements in a 19th century household. Whales are said to have produced “the plastic of the 1800s.” The "bone" of the whale which was most commonly used wasn’t technically a bone, it was baleen, a hard material arrayed in large plates, like gigantic combs, in the mouths of some species of whales. The purpose of the baleen is to act as a sieve, catching tiny organisms in sea water, which the whale consumes as food. As baleen was tough yet flexible, it could be used in a number of practical applications. And it became commonly known as "whalebone." Perhaps the most common use of whalebone was in the manufacture of corsets, which fashionable ladies in the 1800s wore to compress their waistlines. One typical corset advertisement from the 1800s proudly proclaims, “Real Whalebone Only Used.” Whalebone was also used for collar stays, buggy whips, and toys. Its remarkable flexibility even caused it to be used as the springs in early typewriters. The comparison to plastic is apt. Think of common items which today might be made of plastic, and it's likely that similar items in the 1800s would have been made of whalebone. Baleen whales do not have teeth. But the teeth of other whales, such as the sperm whale, would be used as ivory in such products as chess pieces, piano keys, or the handles of walking sticks. Pieces of scrimshaw, or carved whale's teeth, would probably be the best remembered use of whale's teeth. However, the carved teeth were created to pass the time on whaling voyages and were never a mass production item. Their relative rarity, of course, is why genuine pieces of 19th century scrimshaw are considered to be valuable collectibles today. Reference: McNamara, Robert. "Objects Made From the Whaling Industry." ThoughtCo, Jul. 31, 2021, thoughtco.com/products-produced-from-whales-1774070.Whale bone during the 17th, 18th, 19th and early 20th centuries was an important industry providing an important commodity. Whales from these times provided everything from lighting & machine oils to using the animal's bones for use in corsets, collar stays, buggy whips, and many other everyday items then in use.Whale jaw bone one side, long & curved with advanced stage of calcification off white to grey.None.warrnambool, flagstaff-hill, flagstaff-hill-maritime-museum, maritime-museum, shipwreck-coast, flagstaff-hill-maritime-village, whale bones, whale skeleton, whales, whale bone, corsets, toys, whips, whaleling industry, maritime fishing, whalebone -
 Parks Victoria - Point Hicks Lightstation
Parks Victoria - Point Hicks LightstationLid, ship tank
The heavy cast iron, round lid was originally fastened into a large, riveted metal box, known as a ship tank. It has the name ‘John Bellamy London’ cast in capitals in a continuous circle on the outer edge of the lid face, and the words ‘Byng St Millwall’ on the inner circle. , of Millwall, London, manufactured boilers and ship tanks from the 1860s to the 1930s and came from a family of tank makers who began manufacturing tanks some time before 1856. Ship tanks were invented in 1808 by notable engineer, Richard Trevithick and his associate John Dickinson. Their patent obtained the same year described the tank’s superior cubic shape that allowed it to fit squarely as a container in vessels and thus use space efficiently, while its metal fabric preserved and secured its liquid or solid contents from damage. The containers revolutionised the movement of goods by ship and made wooden casks redundant. Research by Michael Pearson has determined that they were carried on passages to Australia from at least the 1830s conveying ships’ victuals and water storage, as well as general goods heading for the colonies. Pearson found photographic evidence of their use in the 1860s, and by the 1870s they appeared to be in common use. lids surviving from containers indicate that nearly all the tanks transported to Australia came from London manufacturers. It was usual for the brand name to also feature as a stencil on the tank but in most cases this eventually wore off. A tank without its original stencil survives at Wilsons Promontory. Tanks transporting ‘drinking water or perishable dry goods were hermetically sealed by the use of the tightly fitting lid with a rubber sealing ring ‘which was screwed tight with the aid of lugs cast into the lid and wedges cast into the rim of the loading hole’. The raised iron rod welded across the outer face of many lids such as the Bellamy example, allowed for screwing the lid tight. Once in the colonies, the ship tanks were often recycled and adapted for many resourceful uses such as packing cases, dog kennels, water tanks, oil containers and food stores and this invariably led to the separation of the lid and tank. The Bellamy lid could have been salvaged from a shipwreck but is more likely to have to have originated from a recycled tank that was brought to the lightstation for water storage purposes. Pearson writes that: Ship tanks show up at a wide range of sites, many of them isolated like lighthouses. They were, I think, usually taken there for the purposes they filled, usually water storage, as they were readily available, relatively light to transport, and probably very cheap to buy as second-hand goods containers. In rural areas they may have been scavenged for their new uses from local stores, to whom goods were delivered in them. Parks Victoria has identified five tank lids in the lightstation collections covered by this project. In addition to the Bellamy lid at Point Hicks, they include a Bow brand lid at Point Hicks and another at Cape Otway, unidentified lids at Cape Otway and Wilsons Promontory. Pearson and Miles Lewis have each recorded two versions of the Bellamy trade name on the lids; one being ‘John Bellamy Byng St. London’; the other, ‘John Bellamy Byng St. Millwall London’. The Point Hicks lid has the second version of the name, as do other examples in Victoria that Lewis has identified at Illawarra, Toorak; Warrock homestead, Casterton; Eeyeuk homestead, Terang; Ward’s Mill, Kyneton; and Boisdale homestead near Maffra, and in NSW at Ayrdale Park, Wolumla; and Bishop’s Lodge, Hay. Pearson’s list includes the same lids in NSW at Tumbarumba; the Quarantine Station, Sydney; Willandra Station; Bedervale, Braidwood; Gunnedah Museum; Walla Walla and Macquarie Island. The Point Hicks lid is currently stored in the lighthouse although it is unlikely that its use had any association with this building. The lid is in good condition and retains the central bung. Pearson notes that ‘surviving lids are far less numerous than the tanks themselves, presumably because the uses to which the tanks were put did not require the lid to be retained’.347 The Bellamy ship tank lid has first level contributory significance for its historic values. Circular cast-iron disc with raised outer ridge with inscription. It also has an inner depression with inscription. Two metal sections form handles over inner depression. Hole in middle of disc.Around perimeter of outer edge "JOHN BELLAMY LONDON" Around inner area "BYNG ST MILLWALL" -
 Flagstaff Hill Maritime Museum and Village
Flagstaff Hill Maritime Museum and VillageDomestic object - Strainer
Take a stroll through the average beverage aisle in your supermarket, and you might get the impression that tea has always come in small boxes with disposable tea bags. But before those easy to come by boxes, there was the rich and intriguing history of the tea strainer, a beautiful little tool that has helped our ancestors enjoy loose leaf tea for hundreds of years. Enjoying loose-leaf tea, and becoming familiar with this tool, can help spark an appreciation for your tea strainer and infuser collection, or simply inspire you to grow one. Documentation of tea tools such as the tea strainer appear in ancient history, the earliest models were likely made of bamboo, and later evolved into stainless steel, sterling silver, china, porcelain, silicon, and linen. During the Tang Dynasty in China, a small book called “Classic of Tea” was written describing tea utensils, and they were made to help Buddhist monks keep living things (such as small bugs) out of the drinking water. However, using a tea tool to keep run away tea leaves out of a cup did not become a cited use of the strainer until the 17th century when Dutch merchants made tea more readily available to those outside of the Chinese dynasty. British royals then increased the popularity of tea as their preferred beverage, and it was not long before a newfound fanaticism for tea in Great Britain spread to the American colonies, as did a growing demand for products that could separate loose tea leaves from liquid with ease and flair. Why did people use a strainer to separate out tea leaves in Great Britain and not in China? While the method of serving tea from a teapot with the tea loose in the pot was a practice used in both countries, the reason China may not have required a tool to remove leaves from their cup likely had to do with the types of tea leaves they were producing. The British owned tea plantations, in countries such as India, produced finer black tea leaves that did not require as much space to expand inside of a tea pot, where as the leaves prepared on the Chinese plantations would expand far more in the pot, and were therefore less likely to land or be bothersome inside a tea cup. This common approach to serving tea with smaller tea leaves required a solution to avoid ending up with a cup, and mouth, full of tea leaves. The obvious solution was a strainer basket. In the Victorian era, tea strainer baskets, similar to those still used in tea parlors today, were made to sit on top of the cup to capture the leaves when pouring the tea from a tea pot into the individual cups. Another solution was a tea-removing device called a mote spoon. Mote spoons act as search and rescue spoons to remove tea leaves from individual teacups. The tea would be brewed loose in the teapot, so any tea that ended up in the cup could be removed with a long handled spoon with holes in the spoon to remove rogue tea leaves and keep the steeped water in the cup. The handle also helped keep the teapot spout free of leaves and could help unclog any leaves trapped when pouring. Stainless steel tea strainers and tea infusers gained popularity in the late 19th century. Big name tea strainer producers, such as Tiffany and Gorham, could use fine silver to create quality, heavy, and sturdy strainers, for those who could afford it. There were many varieties of strainers at that time, but it was more likely that smaller designers who could not afford to mass-produce these quality strainers out of silver made them into unique shapes to attract consumers with lighter wallets. And borne was the tea strainer we are accustomed to today. Things took an unexpected turn for the tea strainer in the early 1900s when Thomas Sullivan, a tea merchant, shipped out tea samples in small silk bags. Customers did not realize that they were supposed to remove the tea from the bags, and instead boiled the tea, bag and all! The convenience of tossing out the leaves is obvious, and the popularity of tea bags is still seen today. Most premium bags of tea we are accustomed to today are frequently packaged loose for consumption, and when they are available in bags, the leaves are often crowded and do not have enough space to expand. While pyramid tea bags have become a more recent solution to this problem, due to the additional space at the top of the bag, enjoying a variety of quality tea is easier with a tea strainer in your arsenal. Besides, with the wide variety of strainers for your cup or pot in versatile materials such as mesh, silver, or a novelty silicone cartoon shape, loose tea can still reign supreme. Tea strainers sometimes do require more cleanup and measuring, but the experience and quality is always worth the effort. Besides, strainers also allow for mixing favorite tea blends together for an extra dose of delicious creativity! https://www.teamuse.com/article_170413.html The strainer provided the convenience of separating the tea leaves for disposal later.Metal strainer, bowl shaped, with mesh and twisted wire handle.Noneflagstaff hill, warrnambool, shipwrecked-coast, flagstaff-hill, flagstaff-hill-maritime-museum, maritime-museum, shipwreck-coast, flagstaff-hill-maritime-village, strainer -
 Flagstaff Hill Maritime Museum and Village
Flagstaff Hill Maritime Museum and VillageCeramic - Tile, circa 1878
This Minton floor tile is from the wreck of the LOCH ARD along with other examples of this manufacture recovered from the wreck site and form part of the collection at Flagstaff Hill. The iron-hulled clipper ship from the Loch Line was heading for Port Phillip from London when it ran into the cliffs of Mutton Bird Island near Port Campbell and was wrecked on June 1st, 1878. The LOCH ARD was laden with high-value cargo including luxury goods intended for display at the Melbourne International Exhibition in 1880. One notable survivor from the ship’s freight manifest was the well-packed Minton porcelain peacock, a two-metre-high ceramic masterpiece of vivid glazed colours. The almost total loss of life and property from the LOCH ARD registered as a shocking tragedy for the Colony of Victoria, at a time when social confidence and economic optimism were otherwise high. The wealth generated from Gold and Wool was increasingly being spent on grandiose private residences and imposing public buildings. The demand for quality furnishings and fittings was therefore strong. Among the products consigned to burgeoning colonial markets by the Milton Pottery at Stoke upon Trent, were their new range of colourfully patterned but very durable floor tiles – ideal for the high-traffic spaces in the large civic buildings then being constructed in Australia and America. These new floor tiles were “encaustic”, meaning that their designs and colours were encased “within” the depth of the tile. Rather than their decorative patterns being glazed onto the surface of the tile, their inlaid designs were created during the manufacturing process, as “coloured slips” (or liquid clay) were poured into a deep pre-moulded casting. When fired, the resulting tile was colour-fast and design-fast. A brief history of the Loch Ard (1873-1878): - The sailing ship Loch Ard was one of the famous Loch Line ships that sailed from England to Australia. Barclay, Curdle and Co. built the three-masted iron vessel in Glasgow in 1873. It had sailed three trips to Australia and one trip to Calcutta before its fateful voyage. Loch Ard left England on March 2, 1878, under the command of recently married, 29-year-old Captain Gibbs. It was bound for Melbourne with a crew of 37, plus 17 passengers. The general cargo reflected the affluence of Melbourne at the time. Onboard were straw hats, umbrellas, perfumes, clay pipes, pianos, clocks, confectionery, linen and candles, and a heavier load of railway irons, cement, lead and copper. Other cargo included items intended for display in the Melbourne International Exhibition of 1880. The Loch Ard had been sailing for three months and was close to its destination on June 1, 1878. Captain Gibbs had expected to see land at about 3 am but the Loch Ard ran into a fog that greatly reduced visibility and there was no sign of land or the Cape Otway lighthouse. The fog lifted at 4 am and the sheer cliffs of Victoria's west coast were much closer to them than Captain Gibbs expected. He tried to manage the vessel but failed and the ship struck a reef at the base of Mutton Bird Island, near Port Campbell. The top deck loosened from the hull, and the masts and rigging crashed down, knocking passengers and crew overboard. The lifeboat was launched by Tom Pearce but crashed into the side of Loch Ard and capsized. He clung onto its overturned hull and sheltered under it. He drifted out to sea and the tide brought him back to what is now called Loch Ard Gorge. He swam to shore and found a cave for shelter. A passenger, Eva Carmichael, had raced onto the deck to find out what was happening and was confronted by towering cliffs above the ship. She was soon swept off the ship by a huge wave. Eva saw Tom Pearce on a small rocky beach and yelled to attract his attention. He swam out and dragged her to the shelter of the cave. He revived her with a bottle of brandy from a case that had washed up on the beach. Tom scaled a cliff in search of help and followed some horse hoof prints. He came from two men from Glenample Station, three and a half miles away. He told the men of the tragedy and then returned to the gorge while the two men rode back to the station to get help. They reached Loch Ard Gorge and took the two shipwreck survivors to Glenample Station to recover. Eva stayed at the station for six weeks before returning to Ireland by steamship. In Melbourne, Tom Pearce received a hero's welcome and was presented with a medal and some money. Of the 54 crew members and passengers on board, only two survived: the apprentice, Tom Pearce and the young woman passenger, Eva Carmichael, who lost her family in the tragedy. One of the most unlikely pieces of cargo to have survived the shipwreck was a Minton porcelain peacock - one of only nine in the world. The peacock was destined for the Melbourne International Exhibition in 1880. It had been well packed, which gave it adequate protection during the violent storm. Today, the Minton peacock can be seen at the Flagstaff Hill Maritime Museum in Warrnambool. From Australia's most dramatic shipwreck, it has now become Australia's most valuable shipwreck artefact and is one of very few 'objects' on the Victorian State Heritage Register. The Minton floor tile is significant for its hard-wearing yet attractive design. The shipwreck of the LOCH ARD is of State significance. Victorian Heritage Register S417. Flagstaff Hill’s collection of artefacts from LOCH ARD is significant for being one of the largest collections of artefacts from this shipwreck in Victoria. It is significant for its association with the shipwreck, which is on the Victorian Heritage Register (VHR S417). The collection is significant because of the relationship between the objects, as together they have a high potential to interpret the story of the LOCH ARD. The LOCH ARD collection is archaeologically significant as the remains of a large international passenger and cargo ship. The LOCH ARD collection is historically significant for representing aspects of Victoria’s shipping history and its potential to interpret sub-theme 1.5 of Victoria’s Framework of Historical Themes (living with natural processes). The collection is also historically significant for its association with the LOCH ARD, which was one of the worst and best-known shipwrecks in Victoria’s history. A square Minton floor tile with a black and apricot pattern against a chocolate brown background. There is a large chip missing. This decorative floor tile was recovered from the shipwreck of the LOCH ARD. On the back, or base, of the tile is inscribed the number “46” and the letters “Minton & Co Patent Stoke upon Trent”.flagstaff hill, warrnambool, shipwrecked coast, flagstaff hill maritime museum, maritime museum, shipwreck coast, flagstaff hill maritime village, great ocean road, loch line, loch ard, captain gibbs, eva carmichael, tom pearce, glenample station, mutton bird island, loch ard gorge, encaustic tile, melbourne international exhibition, floor tile, minton floor tile -
 Coal Creek Community Park & Museum
Coal Creek Community Park & MuseumBottle, glass, c. 1885
150 years of experience and commitment. Norwegians have been producing and exporting cod liver oil for more than 1000 years. But it was not before 1645 it was reported that cod liver oil could be used to prevent and cure disease. At the end of the 18th century the first scientific article was published to support this. In the middle of the 19th century, the pharmacist Peter Möller observed that people along the west coast of Norway consuming cod liver oil regularly were rarely ill. He dedicated himself to finding out how this healthy liquid could be produced with better taste and pureness at a lower price. He developed a method of using steam to extract the oil from fresh cod livers. Based on this technological advance, the company Peter Möller was founded in 1854 in Lofoten on Norway’s arctic coast, where you find pure, cold, clean seas and high quality raw material. Peter Joachim Möller (1793-1869) At first Möller’s Cod Liver Oil was believed to be a good source of vitamin D and A, and the health benefits were associated with these vitamins. Peter Möller believed, however, that there were other significant benefits from fatty acids and other ingredients in the cod liver oil – both known and unknown. Peter Möller was dedicated to understanding more about these benefits. His dedication and commitment is clear in Möller's vision to improve people’s health by delivering the highest quality omega 3 products. Timeline 1793 Peter Möller is born in Røros, Norway 26 April. 1819 Peter Möller travels to Christiania (Oslo) and is employed by the pharmacist Frantz Peckel at the Svane chemist. He is employed on condition that he passes his pharmaceutical exam within one year. 1822 Graduated as a pharmacist with a unanimous first grade and with the award of the Professor's special satisfaction. 1842 Together with professors A. Holst and Chr. Boeck, Peter Möller participates in the commission which develops the first Norwegian Pharmacopoeia. 1853 Peter announces his method to cod liver oil works along the coast. He equips cod liver oil factories with new equipment in Lofoten, Ålesund and Kristiansund. The facility outside Ålesund is the most important for testing the method. 1854 The Peter Möller company is established as production has started at the three factories. Sales are lower than anticipated even though the quality is considerably better with the new method. The consumers of cod liver oil had been used to the fact that “good medicine must taste bad” and would not believe that the new and better quality was as healthy. Therefore, the following years are used to introduce consumers to the product, and also to convert more producers to the new method. 1869 Peter Möller dies. There are 70 cod liver oil steamers which use his steam rendering method, and 5000 barrels are produced every year. Möller’s company increases the quality by better routines for quality controls. 1870 Severin A Heyerdal, Möller’s son-in-law, assumes the leadership of the firm after Peter's death. He continues the work by improving the quality of the cod liver oil. The goal was to make it as pure and unaltered as in the liver. At this time, Möller had already started selling its product in the USA. In 1870, WH Schieffelin & Co. ("The oldest drughouse in America") was engaged by Peter Möller in the USA. 1881 Frantz Peckel Möller assumes the leadership of the Peter Möller company. He saw it as his duty to further the work on cod liver oil, and through a combination of solid scientific education and an eminent sense of the great mercantile possibilities, he made Möller’s cod liver oil the number one in the world market. 1914 The first world war leads to Möller’s bottled cod liver oil being shut out of the export market. However, domestic sales are good. 1924 The subsidiary Møystad Möller & Co. is established for bulk exports and the Association of Medicinal Cod liver oil Exporters is established in Bergen in 1925. 1925/26 The green bottles are introduced. Medicinal cod liver oil exports remain almost constant, while total Norwegian cod liver oil exports increase. 1938 The factory on the Løren grounds in Oslo, Norway is built. The factory is in the same place today. Peter Möller’s Pharmaceutical Laboratorium A/S is also established to separate out the scientific business. Investment is made in a new facility for refining and bottling veterinary cod liver oil, and increased production of industrial cod liver oil. 1940 The outbreak of the 2nd world war sees exports fall dramatically, while cod liver oil’s significance as a dietary supplement receives increased attention. Domestic sales increase strongly. 1945 After the war, medicinal cod liver oil retains its high status as an important dietary supplement in the “rebuilding" of the country. Cod liver oil becomes an ”emergency product in ravaged areas where the supply situation is difficult. Competition from other countries such as the USA, England and Iceland increases, and Norway no longer dominates the market. 1983 Möller’s cod liver oil in capsule form is launched and palatable cod liver oil is launched. 1990 Peter Möller A/S merges with Orkla Borregaard A/S (now ORKLA) 2005 Peter Möller merges with CollettPharma. The new company is called MöllerCollett. 2007 Merger between MöllerCollet and DanskDroge. The new company is called Axellus. Oval in section with a thin neck, mauve tinted clear glass bottle with text embossed on side.On side : 'P.MOLLER', 'OL JECOR', 'GADOR VER', 'CHRISTIANIA'.cod liver oil, norway, peter moller, christiana, oslo -
 Melton City Libraries
Melton City LibrariesBook, Frederick Myers work notebooks/diaries, 1923-1933
FREDERICK THOMAS MYERS Born 20th February 1877 at Melton Married Martha Mary Watson 30th April 1908 at the Manse 101 Gore Street Fitzroy according to the rites of the church (Cong) – Congregational. Died Bacchus Marsh and District War Memorial Hospital 30th April 1963 Frederick lived in Melton all his life. Work Notebooks/ diary 1901 – 1905 No 1 1901 Shearing Tally Rockbank, 1901, 1902. Lists 3 Combs 6.0, 12 Cutters 5.0, Tab 1.6 Jongebloed account – Tobacco, matches sardines. Shearing shed 1904 Tuppal 20 Aug. Daily Tally of sheep shorn Total 1730 E C Shopping list - 6 pages of household items, Leader, Age and Weekly Times Tomato Sauce- recipe written in ink Toolern Road Hours worked 35 ½ Reverse order from back cover – Harwood 29th Aug 1902 Shearing Tally 1083 November 1902 - List of dates Mon- Sat. Nov 3rd - Fri Dec 5th E Chalmer. Tally of hours worked. Jan 1903 Thur 1st - 31st Toohey Keilor Road, Feb – March 14th Payment L75:4:9 J Walker April 1903 – 23rd 18 Cutters 7:6 5 Combs 10:0 2 Tab 3:0 Ticket 15:0 Total 1:15:6 Tucker 3:12:6 Other pages are calculations related to hours worked and amounts The notebook contains 26 pages with some blank and other torn out. It measures 2 ½ x 4 inches with a hard black cover. FREDERICK MYERS Workbook/ Diary No 2 Opening 5 pages – Gottfried Jongebloed account - paid 2/11/04 List of grocery and household items. E Chalmer -Pr Boots, 8:9 Tab 1:8 Leader 3’ Reverse order Eynesbury 7th Oct 1904 Shearing Tally 1635 Tucker payment L1:18:6 Tuppal 20th Aug 1904 Tally 1636 [ 4 weeks and 4 days] Feb 1905 Melton 1st – 13, Toolern Rd 14th , April Braybrook Rd. Melton town, G Hobbs Boundary Rd, Melton May G Hobbs 2 pages of food expenses and amount paid Simpson & Co – Butcher account and amount paid Mon 5th –July 22nd Toohey days and hour worked 34 yards Tuppal 20th Aug additional list 1735 Eynesbury 3rd Oct additional list 2093 Diagram 3 drawings in ink –a type of tool Nov 21 1904 List Dunmore Stn Orford Vic Barwidgee Stn Caramut Vic Wharparanna Stn via Tocumural N.S.W. Puckawidgee vis Aug Deniliquin N.S.W. Bundure Stn via Aug Jerilderie Trawalla Ballarat Oct Woorywooryte Oct Vic Yancannia Bourke July Pretty Tower Stn Beaufort November Size of Notebook 2 ¼ x 5 inches. WORK NOTEBOOK 1909, 1910 Wandook 17th Aug 1909. 21 days of shearing 1248 Total. Payment L14 ?? Boomanoomana 23 Sept 1909. 17 days shearing 1124 Total. L13.12.9 Eynesbury 20th Oct 1909. 17 days shearing 1587 Total L17. 13.0 Cobran Aug 13 1910 Studs Rams Total 3397 List of expenses includes 12 cutters, 3 combs, Union ticket, matches, tobacco stamps. Miller, J Egan. Eynesbury Oct 1910 Total 1467 Lal Lal Nov 21 Total 1172 For Backache Tincture of Gentian Compound one ounce Syrup of Rhubarb one ounce Liquid Barkola Compound one ounce Syrup of Ginger five ounce Dose a teaspoonful after each meal and one at bedtime John Collins, Woodside Via Yarram Yarram Mr Fisken Lal Lal Via Yendon Woodlawn Deniliquin 15th October Oct 12th 1911 Eucla Jarrett Barfold 21,000 7? Shearers Clark Moir Estate 10th Aug 1910-1911 Income amounts Shearing income. Mc Donald – thrasher Keating Toohey Cobran 1911 Studs and Rams 1793 Cobran 14th Aug per man 1500 Golf Hill 26th Sept per man 1900 Cobran 14th Aug Cut out 23rd Sept 2368 payment L21..19..4 [after expenses] Spring Plains Oct 2nd 1022 Total Barfold 2636 Total Chaffcutting J.B. Loft Feb 17th - 31 ½ hours John Minns Toohey Started Deep Creek Whelahan Ballarat Rd May 25th Mr J Walker Koohnamu Stn via Junta S.A. N Aitken Bringlibit via Kyneton Income list 1911 to 1912 and amounts earned Cobran L29..0..9 , Spring Plains Barfold L42..6..3, John Minns L4..8..0, J.B. Loft L3..0..6, D Whelahan L22.. 6..0, E Toohey L15.. 1..0, = total L123..2..6 John Minns 12..0, JB Loft 4.. D Whelahan L10..2..0, Barrie L11..5. Total Amount L145..5..6. Cobran 1912 15th Aug 1922 total Rams double. Expenses include 12 + 6 Cutters, 2+2+2 Combs, Political fund, Ticket, Mess Account, Hut Keeper, Hospital, Cook Total L11..8..3. Station Rd - L11..2..9. Golf Hill Sept 26 Cut out 31 Oct Total 2332 Lal Lal Nov 6th 19th 1912 6 days Total 1066 Contracts – Cameron, Barrie, McKay, Toohey, Stn Rd ? Cobran 1913 – Aug 14- 1913 Total 1621 Cobran 1621 Kyneton Park 515 Golf Hill 2144 Lal Lal 1066 Total 5,346 1913 – 1914 Income 5, 350 sheep Local contracts E. Barrie, Greig, Weir, S Barrie, Jongebloed, Burton, E Toohey, Total L133..3..0. Trench & Moran L19..8..0. End of front section of Notebook Work notebooks belonging to Frederick Myers local identities -
 Flagstaff Hill Maritime Museum and Village
Flagstaff Hill Maritime Museum and VillageFunctional object - Dish
A cylindrical clear glass crystallising dish with a spout. Designed to facilitate the slow evaporation of a liquid, leaving behind solid crystals. The crystallising dish was donated to Flagstaff Hill Maritime Village by the family of Doctor William Roy Angus, Surgeon and Oculist. It is part of the “W.R. Angus Collection” includes historical medical equipment, surgical instruments and material once belonging to Dr Edward Ryan and Dr Thomas Francis Ryan, (both of Nhill, Victoria) as well as Dr Angus’ own belongings. The Collection’s history spans the medical practices of the two Doctors Ryan, from 1885-1926 plus that of Dr Angus, up until 1969. ABOUT THE “W.R.ANGUS COLLECTION” Doctor William Roy Angus M.B., B.S., Adel., 1923, F.R.C.S. Edin.,1928 (also known as Dr Roy Angus) was born in Murrumbeena, Victoria in 1901 and lived until 1970. He qualified as a doctor in 1923 at University of Adelaide, was Resident Medical Officer at the Royal Adelaide Hospital in 1924 and for a period was house surgeon to Sir (then Mr.) Henry Simpson Newland. Dr Angus was briefly an Assistant to Dr Riddell of Kapunda, then commenced private practice at Curramulka, Yorke Peninsula, SA, where he was physician, surgeon and chemist. In 1926, he was appointed as new Medical Assistant to Dr Thomas Francis Ryan (T.F. Ryan, or Tom), in Nhill, Victoria, where his experiences included radiology and pharmacy. In 1927 he was Acting House Surgeon in Dr Tom Ryan’s absence. Dr Angus had become engaged to Gladys Forsyth and they decided he further his studies overseas in the UK in 1927. He studied at London University College Hospital and at Edinburgh Royal Infirmary and in 1928, was awarded FRCS (Fellow from the Royal College of Surgeons), Edinburgh. He worked his passage back to Australia as a Ship’s Surgeon on the on the Australian Commonwealth Line’s T.S.S. Largs Bay. Dr Angus married Gladys in 1929, in Ballarat. (They went on to have one son (Graham 1932, born in SA) and two daughters (Helen (died 12/07/1996) and Berenice (Berry), both born at Mira, Nhill ) According to Berry, her mother Gladys made a lot of their clothes. She was very talented and did some lovely embroidery including lingerie for her trousseau and beautifully handmade baby clothes. Dr Angus was a ‘flying doctor’ for the A.I.M. (Australian Inland Ministry) Aerial Medical Service in 1928 . Its first station was in the remote town of Oodnadatta, where Dr Angus was stationed. He was locum tenens there on North-South Railway at 21 Mile Camp. He took up this ‘flying doctor’ position in response to a call from Dr John Flynn; the organisation was later known as the Flying Doctor Service, then the Royal Flying Doctor Service. A lot of his work during this time involved dental surgery also. Between 1928-1932 he was surgeon at the Curramulka Hospital, Yorke Peninsula, South Australia. In 1933 Dr Angus returned to Nhill and purchased a share of the Nelson Street practice and Mira hospital (a 2 bed ward at the Nelson Street Practice) from Dr Les Middleton one of the Middleton Brothers, the current owners of what previously once Dr Tom Ryan’s practice. Dr Tom and his brother had worked as surgeons included eye surgery. Dr Tom Ryan performed many of his operations in the Mira private hospital on his premises. He had been House Surgeon at the Nhill Hospital 1902-1926. Dr Tom Ryan had one of the only two pieces of radiology equipment in Victoria during his practicing years – The Royal Melbourne Hospital had the other one. Over the years Dr Tom Ryan had gradually set up what was effectively a training school for country general-practitioner-surgeons. Each patient was carefully examined, including using the X-ray machine, and any surgery was discussed and planned with Dr Ryan’s assistants several days in advance. Dr Angus gained experience in using the X-ray machine there during his time as assistant to Dr Ryan. When Dr Angus bought into the Nelson Street premises in Nhill he was also appointed as the Nhill Hospital’s Honorary House Surgeon 1933-1938. His practitioner’s plate from his Nhill surgery is now mounted on the doorway to the Port Medical Office at Flagstaff Hill Maritime Village, Warrnambool. When Dr Angus took up practice in the Dr Edward and Dr Tom Ryan’s old premises he obtained their extensive collection of historical medical equipment and materials spanning 1884-1926. A large part of this collection is now on display at the Port Medical Office at Flagstaff Hill Maritime Village in Warrnambool. In 1939 Dr Angus and his family moved to Warrnambool where he purchased “Birchwood,” the 1852 home and medical practice of Dr John Hunter Henderson, at 214 Koroit Street. (This property was sold in1965 to the State Government and is now the site of the Warrnambool Police Station. and an ALDI sore is on the land that was once their tennis court). The Angus family was able to afford gardeners, cooks and maids; their home was a popular place for visiting dignitaries to stay whilst visiting Warrnambool. Dr Angus had his own silk worm farm at home in a Mulberry tree. His young daughter used his centrifuge for spinning the silk. Dr Angus was appointed on a part-time basis as Port Medical Officer (Health Officer) in Warrnambool and held this position until the 1940’s when the government no longer required the service of a Port Medical Officer in Warrnambool; he was thus Warrnambool’s last serving Port Medical Officer. (Masters of immigrant ships arriving in port reported incidents of diseases, illness and death and the Port Medical Officer made a decision on whether the ship required Quarantine and for how long, in this way preventing contagious illness from spreading from new immigrants to the residents already in the colony.) Dr Angus was a member of the Australian Medical Association, for 35 years and surgeon at the Warrnambool Base Hospital 1939-1942, He served with the Australian Department of Defence as a Surgeon Captain during WWII 1942-45, in Ballarat, Victoria, and in Bonegilla, N.S.W., completing his service just before the end of the war due to suffering from a heart attack. During his convalescence he carved an intricate and ‘most artistic’ chess set from the material that dentures were made from. He then studied ophthalmology at the Royal Melbourne Eye and Ear Hospital and created cosmetically superior artificial eyes by pioneering using the intrascleral cartilage. Angus received accolades from the Ophthalmological Society of Australasia for this work. He returned to Warrnambool to commence practice as an ophthalmologist, pioneering in artificial eye improvements. He was Honorary Consultant Ophthalmologist to Warrnambool Base Hospital for 31 years. He made monthly visits to Portland as a visiting surgeon, to perform eye surgery. He represented the Victorian South-West subdivision of the Australian Medical Association as its secretary between 1949 and 1956 and as chairman from 1956 to 1958. In 1968 Dr Angus was elected member of Spain’s Barraquer Institute of Barcelona after his research work in Intrasclearal cartilage grafting, becoming one of the few Australian ophthalmologists to receive this honour, and in the following year presented his final paper on Living Intrasclearal Cartilage Implants at the Inaugural Meeting of the Australian College of Ophthalmologists in Melbourne In his personal life Dr Angus was a Presbyterian and treated Sunday as a Sabbath, a day of rest. He would visit 3 or 4 country patients on a Sunday, taking his children along ‘for the ride’ and to visit with him. Sunday evenings he would play the pianola and sing Scottish songs to his family. One of Dr Angus’ patients was Margaret MacKenzie, author of a book on local shipwrecks that she’d seen as an eye witness from the late 1880’s in Peterborough, Victoria. In the early 1950’s Dr Angus, painted a picture of a shipwreck for the cover jacket of Margaret’s book, Shipwrecks and More Shipwrecks. She was blind in later life and her daughter wrote the actual book for her. Dr Angus and his wife Gladys were very involved in Warrnambool’s society with a strong interest in civic affairs. He had an interest in people and the community They were both involved in the creation of Flagstaff Hill, including the layout of the gardens. After his death (28th March 1970) his family requested his practitioner’s plate, medical instruments and some personal belongings be displayed in the Port Medical Office surgery at Flagstaff Hill Maritime Village, and be called the “W. R. Angus Collection”.The W.R. Angus Collection is significant for still being located at the site it is connected with, Doctor Angus being the last Port Medical Officer in Warrnambool. The collection of medical instruments and other equipment is culturally significant, being an historical example of medicine from late 19th to mid-20th century. Dr Angus assisted Dr Tom Ryan, a pioneer in the use of X-rays and in ocular surgery.Heavy clear glass pouring dish, with pouring lip possibly used in preparation of pharmaceutical mixtures.None.flagstaff hill, warrnambool, shipwrecked-coast, flagstaff-hill, flagstaff-hill-maritime-museum, maritime-museum, shipwreck-coast, flagstaff-hill-maritime-village, crystallising dish, glass
