Showing 172 items matching "gold processing"
-
 Eltham District Historical Society Inc
Eltham District Historical Society IncPhotograph - Digital Photograph, Alan King, Ore crusher, Golden King mine, Yarrambat, 22 January 2008
Prior to 1929 Yarrambat was known as Tanck's Corner or Reynold's Corner after Frederick Tanck who owned land north of Ironbark Road at the corner of Yan Yean Road, and Thomas Ryenolds who owned the property opposite. Tanck's Corner was at the centre of gold bearing country. Gold was mined here until 1984 when the last operating mine, the Golden King mine in North Oatlands Road closed. It was owned by the Clayton family and in the 1960s was the only private family gold mine in Victoria. Larger mines had their own batteries and stampers to process the ore. Covered under Heritage Overlay, Nillumbik Planning Scheme. Published: Nillumbik Now and Then / Marguerite Marshall 2008; photographs Alan King with Marguerite Marshall.; p23This collection of almost 130 photos about places and people within the Shire of Nillumbik, an urban and rural municipality in Melbourne's north, contributes to an understanding of the history of the Shire. Published in 2008 immediately prior to the Black Saturday bushfires of February 7, 2009, it documents sites that were impacted, and in some cases destroyed by the fires. It includes photographs taken especially for the publication, creating a unique time capsule representing the Shire in the early 21st century. It remains the most recent comprehenesive publication devoted to the Shire's history connecting local residents to the past. nillumbik now and then (marshall-king) collection, battery, clayton family, gold mining, golden king mine, ore crusher, tancks corner. reynolds corner, yarrambat -
 Eltham District Historical Society Inc
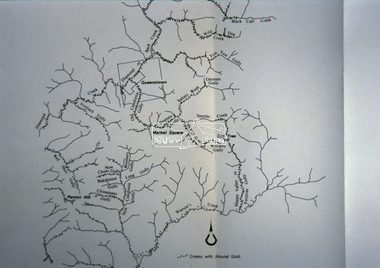
Eltham District Historical Society IncSlide - Photograph, Creeks with Alluvial gold, St Andrews, c.Sep. 1989
... views. Map Creeks Alluvial gold St Andrews Process Date Sep 1989 ...Part of a slide show presentation "Bridges & Waterways of the Shire" by Russell Yeoman to the 13 September 1989 Society meeting. The presentation included slides of historic photos from the Shire of Eltham Pioneers collections as well as several recent views.35mm colour positive transparency (1 of 33) Mount - Agfa CS System grey 8 dotsProcess Date Sep 1989map, creeks, alluvial gold, st andrews -
 Eltham District Historical Society Inc
Eltham District Historical Society IncPhotograph, Tess Justine (Nillumbik Shire Council), Murray's Bridge over the Diamond Creek, Eltham North, 19 Feb 2022
Murray's Bridge over the Diamond Creek on the Diamond Creek Trail just prior to demolition and replacement with a new steel bridge. Heritage advice obtained by Nillumbik Shire Council, following a suggestion by the Eltham District Historical Society (EDHS), is that the original bridge appears to have been a simplified version of the Country Roads Board’s (CRB) standard timber bridge design of the early-to-mid 1920s. In c1990 Murray’s bridge was renovated with three recycled steel girders as part of a bike/pedestrian path in the reserve. During these alterations many parts of the bridge were removed, and some were replaced. Heritage advice indicates the condition of Murray’s bridge is poor. The remaining original parts are all in poor condition, with severe weathering, splitting and rot, especially to the stringers retained on the bridge. Heritage advice is that Murray’s Bridge does not have sufficient significance in the cultural history of the Nillumbik area to warrant inclusion in the Nillumbik Shire Heritage Overlay and also does not have sufficient significance as a rare survivor to warrant inclusion in the Nillumbik Shire Heritage Overlay. There are no indications in the historical record that this site was individually important to the cultural history of this area. EDHS is comfortable with the heritage advice provided to Council and has worked closely on this project with Council. EDHS has suggested some of the removed timbers be used in the vicinity of the bridge for landscaping and possibly seating, so as to retain these remnants close to the site of the original bridge, which is the last old timber bridge along the lower reaches of the Diamond Creek. Mary (Sweeney) Murray and John Wright Murray selected 80 acres, Lot C Section 16 and Lot 5 Section 17 Parish of Nillumbik, under an occupation license in 1866. John died in 1867 and freehold was granted to his son John in 1873. The farm was known as ‘Laurel Hill’. John Junior was an Eltham Shire councillor and sometime president from 1887 up until 1897. He added Lot A Section 16 to the farm in ca1888. John and his younger brother James arranged to rent/purchase Lot B Section 17, across Diamond Creek to the west, in ca1900. It appears that John and James farmed separately for a few years, with a new homestead built for James ad family on the high point of Lot B Section 17 in ca1910. John sold off Lot 5 Section 17 in 1912. When John died in 1912 James took over the land on both sides of the Diamond Creek. The old homestead on the west side of the Creek disappeared. A farm bridge over Diamond Creek from this period may have been located close to the northern boundary of the farm. John Langlands, owner of the farm known as ‘Ihurst’ on the west side of Diamond Creek to the south of the Murray’s land, died in 1907. In 1909 his land was then subdivided into 100 lots to become the ‘Glen Park Estate’. Other similar subdivisions of nineteenth century farms around Eltham in this period included the ‘Franktonia (or Beard’s) Estate’ to the northeast and ‘Bonsack’s Estate’ between Eltham and Greensborough. Soon after the opening of the railway extension line from Eltham to Hurstbridge in 1912, Glen Park and nearby residents including James Murray agitated for a railway station or siding to be located half-way between Eltham and Hurstbridge, so that the Glen Park residents who used the railway daily did not have to walk into the Eltham or Hurstbridge stations. Some believed Coleman’s Corner (opposite Edendale Farm) was an appropriate spot for the platform. James Murray was among those who thought the railway should be located on his land, closer to half-way between Eltham and Hurstbridge stations. The Railways Commissioners warned that the locals would have to fund these works themselves. The Glen Park Estate residents initially had difficulty accessing Eltham by road, with only an old low-level bridge over Diamond Creek at the south end of their estate. A new timber trestle bridge across the creek, now on Wattletree Road, was opened in 1915. Road access to the north was gained in 1927 when the new Murray’s Road, which crossed the Murray’s land, was built. Residents continued to agitate for a Glen Park station. By 1926 the Railways Commissioners’ preferred site was on the Murray’s land. They arranged an estimate of cost of a full-length platform. The estimate was too much for the locals, who in 1928 argued unsuccessfully for a shorter and hence cheaper platform. By 1929 Murray had agreed to donate the land, but the locals would still have to fund the works. Murray decided, unilaterally it would appear, to commence work on a timber trestle road bridge over Diamond Creek to link the new Murray Road to the proposed station. Late in 1929 he stopped work on the bridge, for reasons unknown, but started work again and completed the bridge in 1931. There is no further newspaper evidence of the campaign for the Glen Park station until 1939, when Murray and another local, Mr Maxwell, met the Railways Commissioner. The Glen Park locale now included 45 homes on the west side of the creek and 20 on the Eltham side. Most of the residents used the train every day. The Commissioner remained adamant that only a full-length platform could be built for safety reasons. It appears the campaign dissolved at this point. The increasing move to cars may have had an impact. There is no evidence of Murray’s bridge ever being connected to Murray’s Road, or of it having wide use for any purpose by locals. James Murray died in 1947 and the farm was taken over by his son James (Jim). Jim started to sell off parts of the farm in the 1980s, retaining a few acres around the ca1910 homestead and building a new house there. Recreation reserves were established along the creek. In ca1990 Murray’s bridge was renovated with steel girders as part of a bike/pedestrian path in the reserve. The old farmhouse was demolished in ca2014. * * * A theory posted on local community Facebook groups was that the bridge was built in the 1860s and was built to be more robust than was necessary for the movement of cows from one side of the creek to the other. It was suggested the robustness was necessary to support the weight of gold ore being transferred from a mine on Murray’s land to a railway siding near Murrays Bridge (presumably for transfer and processing at Diamond Creek). Perhaps this may have been one of the motivators for Murray, who really knows? Knowing when mining operations ceased on his land and how that fits the overall timeline would be useful but at the time the bridge was built, local gold production was minimal at best. The known facts are: • The railway line came to Eltham in 1902. • The extension of the railway from Eltham to Hurstbridge was constructed in 1912 so no railway line even existed through Murray's property until 1912 and the Victorian Railways maps at the time show no such siding on Murray’s property. • In 1923 a new company was formed in anticipation of the old Diamond Creek Gold Mine being re-opened. The mine had been previously closed and flooded. It was noted in the press at the time that the mine was within a mile of the railway. Nothing really came of this. • Construction of Murrays Bridge was commenced by James Murray in early 1929 in anticipation of a proposed flag station being nominated on his land, but work ceased shortly afterwards. The proposed flag station was commonly referred to as Glen Park as the residents of the Glen Park Estate wanted Option 1, located near them with the platform adjacent to Colemans corner. This was probably never going to fly as it was virtually in eyesight of Eltham station. Allandale Road was the third option, but the Commissioners' preferred option was No. 2 - on Murray's property. • The Railway Commissioners were not going to finance any such station and the works had to be funded by private landowners and residents, hence Murray investing in this himself. • Murray recommenced work two years later and finished his bridge in 1931 but unfortunately for him, the proposed flag station never eventuated. The bluestone siding you reference may well have been built by Murray as part of the proposed station platform. • Up until then, apart from the Main Road bridge, which was washed away in 1924, virtually all local crossings over the Diamond Creek were low lying bridges – Kaylocks Bridge at Brougham Street, Diamond Street bridge, Glen Park Road bridge. It is expected that Murray also had a low-lying bridge to connect his land either side of the creek. These were all washed away or severely damaged multiple times in the 1920s. Lessons were learnt, and Murrays Bridge appears to have been built in accordance with Country Road Board standards of the time. Flood damage was ongoing, and even more recently constructed raised bridges kept getting washed away, e.g., the new Wattle Tree Road bridge in 1958 just months after completion. Murray’s bridge was reinforced with steel some 30 years ago presumably to provide additional floodwater resistance, given the history of bridges disappearing in floodwaters. • In March 1932 it was reported in the Advertiser that there were still some prospectors operating around Eltham North who apart from further scarring the face of the earth over the previous two years had gained significant experience but little gold - hardly a driving factor for constructing a dedicated railway siding and bridge to transfer gold ore. It is far more probable that James Murray was hoping to have the railway station located on his property and invested his money by building the bridge to lead to it as well as a station platform. Had the station eventuated, it may well have driven up the value of his land for subdivision and new housing estates like the Glen Park Estate. That did not eventuate. Whilst the bridge was indeed old (90 years), the core structure being completed in 1931, it had been modified substantially from original and hence had no significant historic value – i.e., it was not a representative example of its type, construction, and age. Given that the bridge was not worthy of saving, the Eltham District Historical Society with Council’s support, and the Eltham Woodworkers group endeavoured to see what suitable sized timbers were salvageable to fabricate a commemorative seat. Unfortunately, the experts at the Woodworkers group were unable to salvage any suitable length/width timbers to fabricate the seat due to the presence of rot. Last remaining wooden trestle bridge on the Diamond Creek Trail just prior to demolition and replacementBorn digital image (27)diamond creek (creek), diamond creek trail, murrays bridge, ‘laurel hill’, john wright murray, mary (sweeney) murra, john murray jnr, james murray, john langlands, ‘ihurst’, ‘glen park estate’, beard's estate, franktonia, bonsack's estate, glen park estate, glen park railway station -
 Federation University Historical Collection
Federation University Historical CollectionBook - Book - Scrapbook, Ballarat School of MInes: Scrapbook of Newspaper Cuttings, Book 68, March 1995 to May 1995
Collection of newspaper articles related to Ballarat School Of Mines.They cover activities and advertisements for staff. The papers concerned are The Courier, Ballarat, The Australian, The Age ad other region papers over the period of 11 March 1995 to 11 May 1995.Book with yellow cover, front, spiral bound. teaching positions advertised, pre-employment courses, courses available, enrolment for smb courses, ballarat region's workskill, workskill olympics in hairdressing in ballarat, return of paddle steamer, ararat secondary college adopts charter, smb training restaurant, smb horticulture complex, certificate in food processing introduced, plan to relocate senior campus, luke loader pastoral apprentice of the year, students protest workcover policy, closer links with uni needed, geoffrey blainey re writing, program to help child care students, permaculture, early ties with geology, dan daly gold medallist, play therapy in hospital, smb campus link-up, fire threat at smb, james hare apprentice chef, ballarat uni grows, smb maintains record, proposed tafe campus for stawell, education in the 90's, push for rail centre, volunteers vital to community, mining to much more, doug cowles obituary, smb and grampians national park -
 Federation University Historical Collection
Federation University Historical CollectionPhotograph, Ballarat School of Mines Corbould Building
Corbould Hall was named after Ballarat School of Mines alumni WIlliam Corbould. William Corbould was the son of a Ballarat tailor. He attended Ballarat College, and obtained certificates in assaying and metallurgy at the Ballarat School of Mines (SMB) in 1883, studying under the revered Professor Mica Smith. Corbould was not a born student and remembered his first experience at SMB: 'From the Registrar's Office I was led to be introduced to the Professor of Chemistry, one Mica Smith. The initial encounter gave me little encouragement - his large laboratory was filled with hundreds of bottles bearing strange labels with queer symbols on them. My heart sank. At the first opportunity I grabbed my hat and made for the door, but the good professor called me back. I pointed out that I was never any good at school ... so it was no use pretending to be clever enough to understand all those weird symbols! The Professor told me not to worry about that and took me to one of the benches where he found a blowpipe and a charcoal block. Mixing together two powders from bottles on the shelf he transferred a sample to the charcoal and directed the bunsen flame onto it. Soon it began to melt and a white bead appeared in front of my eyes. He then took a test tube and added a little colourless liquid from each of two bottles. A beautiful dark blue colour appeared. My interest was won.' During Corbould's mining career he travelled to Europe twice, and visited most of Australia's main mining fields. Corbould started his career as an assayer at Pinnacle Silver Mine, Silverton, and was then a self-employed assayer at Broken Hill. Corbould became an assayer for the infant BHP mine, and later worked in Kalgoorlie and Coolgardie, including managing Hannan's Reward, the oldest gold mine on the Kalgoorlie gold field. He spent 13 years at the Mount Elliott copper fields as general manager. In 1923, at the age of 57, Corbould went to Mount Isa and reported on options, experimented with new metallurgical processes and floated a company. John Carden of CRA said: 'Corbould was the man who brought Urquhart to Mount Isa. He was the man who made it all possible. He is tremendously important in the Mount Isa story, because he was the first technical man, the first professional man on the scene. He was responsible as I said, for bringing finance to the place, but I think even more importantly he was the first man to recognise the need to put all the little claims in the Mount Isa discovery together. I think perhaps his major contribution to Mount Isa was this amalgamation on the various claims. He recognised that the ore bodies at Mount Isa were not as rich as Broken Hill and they would never have survived had it been fragmented, so he was terribly important.' After completing major financial negotiations for Mt Isa Mine from London in 1927 Corbould remained in Europe where he remained until his death. Corbould was awarded the Legion of Honour of the American Institute of Mining and Metallurigical Engineers for fifty years service. Corbould died at Monaco in 1949 at the age of 82. He bequested 6000 pounds to the Ballarat School of Mines, his will stating 'for the purpose of founding a scholarship to commemorate the memory of the late Alfred Mica Smith'. The accumulated income from this sum provides the Mica Smith travelling scholarship, enabling successful students in mining, metallurgy or chemistry to undertake a year's travelling abroad. The first award was made in 1957. In the same year a general purpose hall at SMB was named the Corbould Hall as a tribute to a distinguished former student and generous benefactor.ballarat school of mines corbould building, corbould hall, corbould building -
 Federation University Historical Collection
Federation University Historical CollectionPamphlet - Promotional brochure, Bachelor of Visual Arts, Graphic Design/Multimedia, c1999
Promoting the Graphic Design/Multimedia program being offered by the University of Ballarat at the Mt Helen Campus. Promoted course as "one of the smallest and arguably the best three year programs of its kind in Australia and the South Pacific region." The brochure lists student awards received including Platinum and Gold in the AGFA International Young Designer Contest, 1999; two meritorious awards in The Art Directors Club Student Awards, New York, USA 1999; Graphis New Talent 1999; two Gold in Souther Cross Packaging Awards, 1998. At time of publication, the School of Arts, Visual Arts reportedly had 210 students with majors in Graphic Design/Multimedia, Ceramics/3D, Painting, Drawing, and Multidiscipline. Minors studies included Printmaking, Photography, 3D, 2D, and Graphic Communication. ___ Course aimed to train "independent, flexible thinkers". The course promised to "Promote creativity, originality and imaginative thinking; Develop self-directed learners, displaying initiative in the formation of ideas and the confidence to construct personal responses; Develop appropriate conceptual, technical and professional skills; Develop the student's critical process: ability to undertake research, and to make informed decisions; Clarify thinking, concepts and understanding and deep knowledge, attitudes and skills enabling the designer to respond to community needs." Studio and working environment described as "one open space with working facilities for approximately 75 students across 3 year levels. The area is divided up into work stations where 1st, 2nd and 3rd year students intermix, allowing a natural interaction. These workstations are configurations of six, consisting of two students from each year level. This reinforces the area's ongoing development with an open ethos and cross-level delivery and learning. This maximises the use of information in order for it to be applied throughout all levels of the learning process, whilst allowing a natural mentor arrangment to be developed for all first year students, " "The open ethos approach also encourages students and staff to freely express their opinions in relation to design via cross-level critiques, whilst allowing for a liberal arts approach and structure to the development of the creative process." "Emphasis is placed on experimentation, innovation, expression and the development of the individual's design philosophies, concepts and style." Also notes the 24 hour access Macintosh laboratory, with 34 Power Macintosh computers, ratio of one for every 2.5 students. Each with a Fujitsu Dyna Magneto Optical drive for file storage and transport. Two Sharp scanners, Phaser Dye-Sublimation Extra Tabloid colour printer and Ricoh A3 colour printer. Two large format printers. Digital and video cameras. Software: Adobe Photoshop, Illustrator, Acrobat; QuarkXpress; Macromedia Freehand; Pagemaker; Premier; Director; 3D Extreme; Sound Eidt, Shockwave, Infinite 3D and After Effects. Approx 4.5 staff, "all of whom are practicing designers. They have a full understanding of industry requirements and trends which assists in the development of industrial contacts when specialists are required." Prospective students interviewed in late Nov/ early Dec, face to face. Present a "comprehensive folio of work", academic records, references. "Selection is determined by the perceived potential of the student, their motivation and reason for study within the field as well as their previous experience in the Visual Arts. Folio work should be representative of the individual's ideas and abilities. Qualities of importance are: originality, innovation, imagination, experimentation and a competent display of the basic skills associated with visual arts [evidence of drawing skills should be included]." Demonstration of GD/MM computer skills an advantage. Students also asked to bring sketch books. Promotional brochure for prospective students. 8pp Double fold brochureuniversity of ballarat, federation university, graphic design, multimedia, bachelor, degree -
 City of Moorabbin Historical Society (Operating the Box Cottage Museum)
City of Moorabbin Historical Society (Operating the Box Cottage Museum)Manufactured Glass, brown bottle 'Penicillin Lozenges', 20thC
F. H. Faulding & Co was a pharmaceutical company founded in Adelaide, South Australia in 1845 by Francis Hardey Faulding 1816 – 1868, a native of Swinfleet, Yorkshire, He arrived in Sydney on the Nabob in February 1842,and travelled on the brig Dorset to Adelaide in May, where he opened a pharmacy at 5 Rundle Street in 1845.The pharmacy flourished, so he purchased a warehouse in Clarence Place in the city and transferred the manufacturing and wholesale arms of the business there. In 1861 he entered into partnership with Luther Scammell (1826–1910).a Yorkshireman, who had received medical training at Guy's Hospital, and arrived in Adelaide in 1849. Faulding died in 1868 and Scammell took over the business, however he was forced to retire in 1889 when the Bank of Adelaide threatened foreclosure after a series of failed mining and pastoral speculations. Two of his sons, Luther Robert Scammell FCS LSA ( 1858 – 1940) and William J. Scammell ( 1856 – 1928) acquired the manufacturing and wholesaling operations, and the business name, in 1888; the retail shops were sold to reduce the debt to the bank.The company expanded under the two brothers and later two sons of each became directors of the company. In June 1921 Faulding & Co. became a private company, with L.R. Scammell as chairman and managing director. He continued to run the firm's affairs until 1935.Two of the Faulding company's major innovations were the development of a process for distillation of eucalyptus oil, and the development of the test for determining the eucalyptol content of the oil. Faulding's success was founded on eucalyptus oil, which formed the basis of an antiseptic marketed as "Solyptol" (for soluble eucalyptus oil). The test became the industry standard, and the British Pharmacopoeia standard method in 1898. Other well-known products were Milk Emulsion (a pleasant alternative to cod-liver oil), Solyptol Soap, (which won a gold medal at the Franco-British Exhibition in London in 1908), Solyptol disinfectant, junket tablets, cordials, essential oils for perfumery and reagents such as Epsom salts, most produced in its factory in Thebarton The Faulding Co. built success around optimising the delivery of oral dosage form drugs. A brown glass bottle with a plastic screw top that contained 'Penicillin Lozenges' made by F.H. Faulding Co. Ltd . AustraliaFAULDING / Penicillin Lozenges / ( Troch. Penicillin B.P.) /......... / F.H.Faulding & Co. Ltd. / Adelaide, Perth, Sydney, / Melbourne, Brisbane on back DIRECTIONS......pharmacy, medicines, f.h. faulding co. ltd, penicillin lozenges, antibiotics, dentists, glassware, bottles, moorabbin, bentleigh, cheltenham -
 Eltham District Historical Society Inc
Eltham District Historical Society IncNegative - Photograph, Members of the Hill family, early Eltham settlers, c.1860
Mrs Georgina Hill (wife of Henry), nee Reynolds (of Research, Vic.) in cap [possibly misidentified by donor - see note below] with Mrs Isaac Hill and her children (left to right) Amelia Hill, (born 1853) Mrs Isaac Hill with baby Isaac (born 1860, Eltham) on her lap. Mary Jane Hill (born 1857, Eltham) seated on Mrs Henry Hill's lap and Bob Hill. The Hill family were early settlers of the Eltham area. Daguerreotype photo enclosed in a leather bound clam shell box with felt lining and gold trim. Donated by Mrs Ivy Edna Hill, 4/1 Bridge Street, Eltham, 4 June 1966 and includes copy of her note identifying the people. Daguerreotypes were one of the first forms of early photographs. They initially appeared in Europe in 1839 and were produced in large numbers to the early 1850s but were superseded by more modern and flexible forms of technology by 1860. The photo was usually formed on a thin copper plate with light sensitve silver iodide. They have a mirror-like appearance and the image itself was mirrored. They were usually inserted into a case or frame made of wood bound in leather or velvet and cost about one guinea in Australia, the equivalent of a week's wages. With the advent of the gold-rush and growing population came an increase in numbers of photographers both studio and travelling. The daguerreotype process was protected by patents and could only result in a single image from which no copies could be made. With new technology involving wet colloidion glass plate negatives and albumen paper prints of which multiple copies could be produced at significantly reduced cost, the dauguerreotype quickly fell out of favour. An accompanying note with the photo written by Edna Hill of 4/1 Bridge Street Eltham dated 4 June 1966 states: "Dear Mr Watson, I think the enclosed old time photograph will be of interest to you. It would have been taken about 1860. The two ladies are the wives of the original pioneers of the Hill family. The one in the cap was the wife of Henry Hill, the other of Isaac Hill. The children are those of Mrs Isaac Hill, and grandchildren to Henry Hill. The little girl on the left is Amelia, the baby Isaac, the second girl is Mary Jane, and the boy on the right is Bob Hill. They grew up tobe Uncles and Aunts of my late husband. I greatly appreciated a letter received a few months ago per Cr Pelling, from the Shillinglaw Cottage Committee. Yours sincerely, Edna Hill" Victorian birth registrations show Mary Jane Hill was born 1857 in Eltham (9879 / 1857) and Isaac Hill at Eltham in 1860 (1972/1860) NOTE: Mrs Isaac Hill was Ellen Fitzsimons (1834-1863), mother to Henry Hill. Mrs Georgina Hill, wife of Henry cannot be the lady in the cap as she was not born till 1864. Georgina Reynolds (1864-1927) married Henry Hill (1862-1948) in 1884. This lady has significant wrinkling of the face, especially around her mouth. It is possible that she is the mother of Mrs Isaac Hill (Ellen Fitzsimons) who was Isabella Fitzsimons (nee Ferguson).This photo forms part of a collection of photographs gathered by the Shire of Eltham for their centenary project book,"Pioneers and Painters: 100 years of the Shire of Eltham" by Alan Marshall (1971). The collection of over 500 images is held in partnership between Eltham District Historical Society and Yarra Plenty Regional Library (Eltham Library) and is now formally known as 'The Shire of Eltham Pioneers Photograph Collection.' It is significant in being the first community sourced collection representing the places and people of the Shire's first one hundred years.Digital image 4 x 5 inch B&W Negsepp, shire of eltham pioneers photograph collection, eltham, hill family, research (vic.), women, amelia hill, bob hill, daguerreotype, early settlers, georgina hill (nee reynolds), isaac hill, mary jane hill, mrs henry hill, mrs isaac hill, ellen hill (nee fitzsimons), isabella fitzsimons (nee ferguson) -
 Kiewa Valley Historical Society
Kiewa Valley Historical SocietyTin Bushells, after 1937
Bushells tea and coffee producers started in Brisbane(1883) and expanded to Sydney, Melbourne(1899), Fremantle and finally to Auckland(1937). Like other Pacific Island sourced condiments Tea and coffee were a lot easier to import than British or European goods. Local Australian industries were starting to develop and grow to overcome the long transportation times and the high costs of goods from traditional suppliers. Bushells Pty Ltd is a prime example.This "Bushells" Coffee tin is a good example of Australian "grown" condiment suppliers serving the "whole" of the Australian marketplace. Rural areas were not neglected and the purchase price for goods were a reasonable levels. Kiewa Valley experienced a population growth from the late 1940's and due to the migrant works in the Kiewa Hydro Scheme the greater use of coffee was initiated. Rural areas, on the whole, where the population was more connected to some degree with a British heritage lineage were predominately tea drinkers. The American film productions, however, screened in Australia post 1950's showing a greater degree of coffee drinking "stars" had a long term effect upon the drinking habits of the rural populous. This round tin of coffee with lid is made from pressed steel lid framed top and bottom. Sturdy cylindrical body is made of cardboard. Paper information label is pasted onto the container with promotional and logo information.In large print and on opposite sides are two manufacturers labels.The topprint and on a diagonal slant in gold letters on a dark blue background is "BUSHELLS" underneath this "PURE" and below this on a red background "COFFEE". On one side in smaller print "EASY TO MAKE" and below this "Use one dessert-spoonful for each cup of coffee desired. Place in the pot and pour over it fresh water briskly boiling. Let stand for five minutes, then strain". Below this "1 IB. net" and underneath is a signature "BUSHELLS" below this in small print "Pty. Ltd." Below this, in a vertical row are"SYDNEY MELBOURNE fREMANTLE AUCKLAND"coffee and tea processors and distributors, hot drink suppliers -
 Kiewa Valley Historical Society
Kiewa Valley Historical SocietyFrame - Photograph
This photograph frame with its decorative floral and Greek patterned boarder was typical of the early 1900's when photography was in the hands of the professional artist. It was in a period before the "instant" photo and required a dark room and processing liquids for development. It was therefor in a time when photographs were "shot" only at important events be they family or public occasions.This photograph frame holds and protects, part of a very significant occasion, the 90th birthday photograph of the matriarch of one of the founding families within the Kiewa Valley. The frame therefore has historical significance.This gold painted aluminium photograph frame has a glass (broken) pane within a formed inlay and two swivel clip toggles. These are installed to allow the photograph to be securely positioned within the correct alignment of the the frame.The metal front plate has seven rivets holding the cloth covered cardboard back frame to the metal front cover. The front part of the frame has sculptured wild flowers and is boarded with a Greek "key" pattern. Half way on the back panel is a rectangular swiveled stabiliser flap. See also KVHS 0093.photograph frame early 1900's, the roper family -
 Eltham District Historical Society Inc
Eltham District Historical Society IncPhotograph - Daguerreotype Photo Case, Members of the Hill family, early Eltham settlers, c.1860
Mrs Georgina Hill (wife of Henry), nee Reynolds (of Research, Vic.) in cap [possibly misidentified by donor - see note below] with Mrs Isaac Hill and her children (left to right) Amelia Hill, (born 1853) Mrs Isaac Hill with baby Isaac (born 1860, Eltham) on her lap. Mary Jane Hill (born 1857, Eltham) seated on Mrs Henry Hill's lap and Bob Hill. The Hill family were early settlers of the Eltham area. Daguerreotype photo enclosed in a leather bound clam shell box with felt lining and gold trim. Donated by Mrs Ivy Edna Hill, 4/1 Bridge Street, Eltham, 4 June 1966 and includes copy of her note identifying the people. Daguerreotypes were one of the first forms of early photographs. They initially appeared in Europe in 1839 and were produced in large numbers to the early 1850s but were superseded by more modern and flexible forms of technology by 1860. The photo was usually formed on a thin copper plate with light sensitve silver iodide. They have a mirror-like appearance and the image itself was mirrored. They were usually inserted into a case or frame made of wood bound in leather or velvet and cost about one guinea in Australia, the equivalent of a week's wages. With the advent of the gold-rush and growing population came an increase in numbers of photographers both studio and travelling. The daguerreotype process was protected by patents and could only result in a single image from which no copies could be made. With new technology involving wet colloidion glass plate negatives and albumen paper prints of which multiple copies could be produced at significantly reduced cost, the dauguerreotype quickly fell out of favour. An accompanying note with the photo written by Edna Hill of 4/1 Bridge Street Eltham dated 4 June 1966 states: "Dear Mr Watson, I think the enclosed old time photograph will be of interest to you. It would have been taken about 1860. The two ladies are the wives of the original pioneers of the Hill family. The one in the cap was the wife of Henry Hill, the other of Isaac Hill. The children are those of Mrs Isaac Hill, and grandchildren to Henry Hill. The little girl on the left is Amelia, the baby Isaac, the second girl is Mary Jane, and the boy on the right is Bob Hill. They grew up tobe Uncles and Aunts of my late husband. I greatly appreciated a letter received a few months ago per Cr Pelling, from the Shillinglaw Cottage Committee. Yours sincerely, Edna Hill" Victorian birth registrations show Mary Jane Hill was born 1857 in Eltham (9879 / 1857) and Isaac Hill at Eltham in 1860 (1972/1860) NOTE: Mrs Isaac Hill was Ellen Fitzsimons (1834-1863), mother to Henry Hill. Mrs Georgina Hill, wife of Henry cannot be the lady in the cap as she was not born till 1864. Georgina Reynolds (1864-1927) married Henry Hill (1862-1948) in 1884. This lady has significant wrinkling of the face, especially around her mouth. It is possible that she is the mother of Mrs Isaac Hill (Ellen Fitzsimons) who was Isabella Fitzsimons (nee Ferguson).Early pioneer settlers of ElthamAntique daguerreotypes in hinged gold frame, glass encased in a small clam-shell box lined with padded red felt and with catchamelia hill, bob hill, early settlers, eltham, hill family, isaac hill, mary jane hill, mrs henry hill, mrs isaac hill, daguerreotype, georgina hill (nee reynolds), research (vic.), sepp, shire of eltham pioneers photograph collection, women, ellen hill (nee fitzsimons), isabella fitzsimons (nee ferguson) -
 Warrnambool and District Historical Society Inc.
Warrnambool and District Historical Society Inc.Functional object - Brick, 94 Merri Street Warrnambool, Circa 1850
The building at 94 Merri St is amongst the earliest buildings in Warrnambool. It was possibly constructed before 1854 and maybe as early as 1848. The original building consisted of four main rooms under a hip roof. The outer walls were masonry , apparently rubble The Heritage Council indcate that on the balance of probabilities this was the general store of Richard Osbourne and John Moffat Chisholm built in 1847-48.The building at 94 Merri Street had historical and architectural significance to the state of Victoria. It was a rare example of surviving, pre separation building with associated interest in its materials and components It is associated with Richard Osbourne who founded the Warrnambool Examiner(1851_1889) and the town’s first historian. Architecturally, the significance of the house lay in the hand -wrought timber framing. All the major timbers in this building had been hand sawn. The Gold rush of the 1850’s saw this process mechanised. A kitchen fireplace in the rear skillion included a large block of Merri Creek Mudstone, a combination of “tufa” and clay that was used in the Warrnambool District as an excellent substitute for fire bricks. The house was demolished in 2011 and items of significance were salvaged, this brick being one. While some bricks were imported, there is evidence that there were a number of brick burning businesses in operation around this time. However it is difficult to ascertain to which group this brick belongs. Red clay rectangular brick. Contains some small gravel like material, possibly ferrous buckshot, grey mortar remnants on side and diagonal crack along one side.warrnambool, 94 merri street, richard osbourne, john moffat chisholm, hand sawn timber, brick, building materials -
 Cheese World Museum
Cheese World MuseumDVD, A Look at our History -Vol.2, 2005
Series of interviews with former employees of Kraft (Allansford) and directors of Warrnambool Cheese and Butter Factory discussing their time and experiences as employees and directors of the factories. Warrnambool Cheese and Butter Company Ltd was established in 1888 and in 1935 developed a relationship with Kraft Foods Australia whereby Kraft leased premises from WCB. The two companies shared the milk collected to make their core products of cheese (Kraft) and butter and milk for domestic use (WCB). This arrangement continued until 1997.Gold DVD in plastic case, B&W cover with colour WCB logo.A Look at our History: Ron Campbell, Steve Heazlewood, Jim Mahony, DJ (Barney) Loganallansford, heazlewood, steve, campbell, ron, mahony, jim, logan, dj (barney), wcb, warrnambool cheese and butter factory company ltd, kraft foods ltd, dairy industry, dairy processors, factories, local history, cairns, peter, cheese manufacture -
 Flagstaff Hill Maritime Museum and Village
Flagstaff Hill Maritime Museum and VillageCeramic - Teapot, First half of the 20th century
Lusterware is a type of pottery or porcelain with a metallic glaze that gives the effect of iridescence. The technique on pottery was first developed in Mesopotamia (modern Iraq) in the early 9th century. It is produced by metallic oxides in an overglaze finish, which is given a second firing at a lower temperature in a "muffle kiln", or a reduction kiln, excluding oxygen. The Lusterware effect is a final coating applied over the ceramic glaze, and fixed by a light second firing, applying small amounts of metallic compounds (generally of silver or copper) mixed with something to make it paintable (clay or ochre). This is then fired in a reducing atmosphere at a temperature high enough to "soften" the glaze from the first firing, and break down the metallic compounds, leaving a very thin ("perhaps 10 or 20 atoms thick") layer that is fused with the main glaze, but is mainly metal. Lusterware normally only uses one colour per piece, and the range is limited a "gold" derived from silver compounds was historically the most common. The process has always been expensive and rather unpredictable, always requiring two firings, and often the use of expensive materials such as silver and platinum. The very thin layer of luster is often delicate, and many types of Lusterware are easily damaged by scratching removing the metallic layer, or by contact with acids. Lusterware has therefore always been for display and occasional use, although by the 19th century it could be relatively cheap. Many pieces show the luster effect only working correctly on parts of the surface, or not at all. An item probably made in Staffordshire UK where this type of pottery was popular in the late 19th century by unknown pottery as the subject item has no marks. The teapot at this time cannot be associated with a historical event, person, or place, provenance regards manufacture is unknown, item assessed as a collection asset given it was produced before 1950.Teapot ceramic ornate copper lusterware abstract floral design handle has a protruding sculptured bird for thumb grip. Nonewarrnambool, flagstaff-hill, flagstaff-hill-maritime-museum, maritime-museum, shipwreck-coast, flagstaff-hill-maritime-village, teapot, tea set, kitchen ware, ceramic, lusterware, pottery, staffordshire uk, pottery finishes -
 Flagstaff Hill Maritime Museum and Village
Flagstaff Hill Maritime Museum and VillageDomestic object - Bowl, Late 19th or early 20th Century
The Process of Making Pottery Decorating, Firing, Glazing, Making, Technical There is a rhythm and flow to clay. It can’t be done all at once! Even the making process! It can take weeks to get everything done, especially if you can only work on your pottery once a week! Even though we have three hour classes, it’s often just not enough time! Here is an overview of some of the processes so you have a bit more grasp on some of the technical stuff! Step One – Design There are SO many ideas out there for making stuff in clay! From delicate porcelain jewellery, through to heavy sculptural work and everything in between. Deciding your direction is sometimes not that easy – when you first start, try everything, you will naturally gravitate to the style that you enjoy! The options and variations are endless and can get a wee bit overwhelming too! Check in with me before you start to ensure your ideas will work, what order you might do things, how you could achieve the look you are seeking and any other technical data required! Step Two – Making Clay is thixotropic. This means that as you work with it, the clay first gets sloppier and wetter, before is begins to dry in the atmosphere. For most things, you simply can’t do all parts of the project at once. An example of work order might look like: Get last weeks work out from the shelves Prepare clay for today’s work – roll your clay, prepare balls for throwing, make the first stage of a pinch pot) Clean up last week’s work and put it on the shelf for bisque firing Check that you have any glazing to do – and do enough of it that you will have time to finish your main project Do the next step of your next project – there might be a further step that can’t be complete immediately, in that case, wrap your work well and put onto the shelves. Letting your work rest for a while can really help keep your work clean and professional looking. Many things require bagging under plastic to keep it ready for work the next week – put your name on the outside of the bag so you can find your work easily. We have stickers and markers. Consider how you want to decorate your work – coloured slip can be applied at a fairly wet stage (remembering that it will make your work even wetter!). Trying to apply slip to dry clay won’t work! If you want to do sgraffito – you will need to keep the work leather hard (a state of dryness where you can still work the clay with a little effort and a little water and care). Step Three – Drying Most of the time your work can go into the rack uncovered to let it dry out for the following week. If you want to continue forming or shaping you will need to double bag your work – put your work on a suitable sized bat and put the bat in a bag so the base of the bag is under the bat, then put another bag over the top of the work and tuck the top of the bag under the bat. If you want to trim (or turn) your thrown work the following week, it should also be double bagged. If your work is large, delicate, or of uneven thicknesses, you should lightly cover your work for drying. When considering the drying process, bare in mind the weather, humidity and wind! The hotter and dryer, the faster things dry and work can dry unevenly in the shelves – this can lead to cracking – another time to lightly cover your work for drying. Step Four – Trimming and Cleaning Up Your work is dry! It is called greenware now and it is at it’s most fragile! Handle everything with two hands. I often refer to soft hands – keep everything gentle and with your fingers spread as much as possible. Try to not pick up things like plates too much, and always with both hands! Before your work can be bisque fired it should be “cleaned up”. You work won’t go into the kiln if it has sharp edges – when glazed, sharp edges turn into razor blades! Use a piece of fly wire to rub the work all over – this will scratch a little so be light handed. Use a knife or metal kidney to scrape any areas that require a bit more dynamic treatment than the fly wire offers! Finally, a very light wipe over with a slightly damp sponge can help soften and soothe all of your edges and dags! Trimming thrown work: If you are planning to trim (or turn) your thrown work (and you should be), make sure you bag it well – your work should be leather hard to almost dry for easiest trimming. Use this step to finish the work completely – use a metal kidney to polish the surface, or a slightly damp sponge to give a freshly thrown look. Wipe the sponge around the rim after trimming, and check the inside of the pot for dags! Trimming slip cast work: Usually I will trim the rims of your work on the wheel the following day to make that stage easier, however you will still need to check your work for lumps and bumps. Last but not least – check that your name is still clearly on the bottom of your work. Step Five – Bisque Firing When the work is completely dry it can go into the bisque kiln. The bisque kiln is fired to 1000°C. This process burns off the water in the clay as well as some of the chemically bound water. The structure of the clay is not altered that much at this temperature. Inside the bisque kiln, the work is stacked a little, small bowl inside a larger bowl and onto a heavy plate. Smaller items like decorations or drink coasters might get stacked several high. Consideration is paid to the weight of the stack and shape of the work. A bisque kiln can fire about one and a half times the amount of work that the glaze kiln can fire. The firing takes about 10 hours to complete the cycle and about two days to cool down. Once it has been emptied the work is placed in the glaze room ready for you to decorate! Step Six – Glazing Decorating your work with colour can be a lot of fun – and time consuming! There are three main options for surface treatment at this stage: Oxide Washes Underglazes Glazes Washes and underglazes do not “glaze” the work – It will still need a layer of glaze to fully seal the clay (washes don’t need glaze on surfaces not designed for food or liquid as they can gloss up a little on their own). Underglazes are stable colourants that turn out pretty much how they look in the jar. They can be mixed with each other to form other colours and can be used like water colours to paint onto your work. Mostly they should have a clear glaze on top to seal them. Oxides are a different species – the pink oxide (cobalt) wash turns out bright blue for instance. They don’t always need a glaze on top, and some glazes can change the colour of the wash! The glazes need no other “glaze” on top! Be careful of unknown glaze interactions – you can put any combination of glaze in a bowl or on a plate, but only a single glaze on the outside of any vertical surface! Glazes are a chemical reaction under heat. We don’t know the exact chemicals in the Mayco glazes we use. I can guess by the way they interact with each other, however, on the whole, you need to test every idea you have, and not run the test on a vertical surface! Simply put, glaze is a layer of glass like substance that bonds with the clay underneath. Clay is made of silica, alumina and water. Glaze is made of mostly silica. Silica has a melting point of 1700°C and we fire to 1240°C. The silica requires a “flux” to help it melt at the lower temperature. Fluxes can be all sorts of chemicals – a common one is calcium – calcium has a melting point of 2500°C, however, together they both melt at a much lower temperature! Colourants are metal oxides like cobalt (blue), chrome (green through black), copper (green, blue, even red!), manganese (black, purple and pink) iron (red brown), etc. Different chemicals in the glaze can have dramatic effects. for example, barium carbonate (which we don’t use) turns manganese bright pink! Other elements can turn manganese dioxide brown, blue, purple and reddish brown. Manganese dioxide is a flux in and of itself as well. So, glazes that get their black and purple colours, often interact with other glazes and RUN! Our mirror black is a good example – it mixes really well with many glazes because it fluxes them – causes them to melt faster. It will also bring out many beautiful colours in the glazes because it’s black colouring most definitely comes from manganese dioxide! Glaze chemistry is a whole subject on it’s own! We use commercial Mayco glazes on purpose – for their huge range of colour possibilities, stability, cool interactions, artistic freedom with the ability to easily brush the glazes on and ease of use. We currently have almost 50 glazes on hand! A major project is to test the interactions of all glazes with each other. That is 2,500 test tiles!!!! I’m going to make the wall behind the wheels the feature wall of pretty colours! Step Seven – Glaze (Gloss or sometimes called “Glost”) Firing Most of the time this is the final stage of making your creation (but not always!) The glaze kiln goes to 1240°C. This is called cone 6, or midrange. It is the low end of stoneware temperatures. Stoneware clays and glazes are typically fired at cone 8 – 10, that is 1260 – 1290°C. The energy requirement to go from 1240°C to 1280°C is almost a 30% more! Our clay is formulated to vitrify (mature, turn “glass-like”) at 1240°, as are our glazes. A glaze kiln take around 12 hours to reach temperature and two to three days to cool down. Sometimes a third firing process is required – this is for decoration that is added to work after the glaze firing. For example – adding precious metals and lustres. this firing temperature is usually around 600 – 800°C depending upon the techniques being used. There are many students interested in gold and silver trims – we will be doing this third type of firing soon! After firing your work will be in the student finished work shelves. Remember to pay for it before you head out the door! There is a small extra charge for using porcelain clay (it’s more than twice the price of regular clay), and for any third firing process! Once your work has been fired it can not turn back into clay for millennia – so don’t fire it if you don’t like it! Put it in the bucket for recycling. https://firebirdstudios.com.au/the-process-of-making-pottery/ The bowl is an example of kitchenware used in the 19th century and still in use today.Bowl white ceramic. Crack on side. Badly stained.Backstamp very faint and unable to be read.flagstaff hill, warrnambool, shipwrecked-coast, flagstaff-hill, flagstaff-hill-maritime-museum, maritime-museum, shipwreck-coast, flagstaff-hill-maritime-village, mixing bowl, food preparation, kitchen equipment, ceramic -
 Flagstaff Hill Maritime Museum and Village
Flagstaff Hill Maritime Museum and VillageDomestic object - Bowl
The Process of Making Pottery Decorating, Firing, Glazing, Making, Technical There is a rhythm and flow to clay. It can’t be done all at once! Even the making process! It can take weeks to get everything done, especially if you can only work on your pottery once a week! Even though we have three hour classes, it’s often just not enough time! Here is an overview of some of the processes so you have a bit more grasp on some of the technical stuff! Step One – Design There are SO many ideas out there for making stuff in clay! From delicate porcelain jewellery, through to heavy sculptural work and everything in between. Deciding your direction is sometimes not that easy – when you first start, try everything, you will naturally gravitate to the style that you enjoy! The options and variations are endless and can get a wee bit overwhelming too! Check in with me before you start to ensure your ideas will work, what order you might do things, how you could achieve the look you are seeking and any other technical data required! Step Two – Making Clay is thixotropic. This means that as you work with it, the clay first gets sloppier and wetter, before is begins to dry in the atmosphere. For most things, you simply can’t do all parts of the project at once. An example of work order might look like: Get last weeks work out from the shelves Prepare clay for today’s work – roll your clay, prepare balls for throwing, make the first stage of a pinch pot) Clean up last week’s work and put it on the shelf for bisque firing Check that you have any glazing to do – and do enough of it that you will have time to finish your main project Do the next step of your next project – there might be a further step that can’t be complete immediately, in that case, wrap your work well and put onto the shelves. Letting your work rest for a while can really help keep your work clean and professional looking. Many things require bagging under plastic to keep it ready for work the next week – put your name on the outside of the bag so you can find your work easily. We have stickers and markers. Consider how you want to decorate your work – coloured slip can be applied at a fairly wet stage (remembering that it will make your work even wetter!). Trying to apply slip to dry clay won’t work! If you want to do sgraffito – you will need to keep the work leather hard (a state of dryness where you can still work the clay with a little effort and a little water and care). Step Three – Drying Most of the time your work can go into the rack uncovered to let it dry out for the following week. If you want to continue forming or shaping you will need to double bag your work – put your work on a suitable sized bat and put the bat in a bag so the base of the bag is under the bat, then put another bag over the top of the work and tuck the top of the bag under the bat. If you want to trim (or turn) your thrown work the following week, it should also be double bagged. If your work is large, delicate, or of uneven thicknesses, you should lightly cover your work for drying. When considering the drying process, bare in mind the weather, humidity and wind! The hotter and dryer, the faster things dry and work can dry unevenly in the shelves – this can lead to cracking – another time to lightly cover your work for drying. Step Four – Trimming and Cleaning Up Your work is dry! It is called greenware now and it is at it’s most fragile! Handle everything with two hands. I often refer to soft hands – keep everything gentle and with your fingers spread as much as possible. Try to not pick up things like plates too much, and always with both hands! Before your work can be bisque fired it should be “cleaned up”. You work won’t go into the kiln if it has sharp edges – when glazed, sharp edges turn into razor blades! Use a piece of fly wire to rub the work all over – this will scratch a little so be light handed. Use a knife or metal kidney to scrape any areas that require a bit more dynamic treatment than the fly wire offers! Finally, a very light wipe over with a slightly damp sponge can help soften and soothe all of your edges and dags! Trimming thrown work: If you are planning to trim (or turn) your thrown work (and you should be), make sure you bag it well – your work should be leather hard to almost dry for easiest trimming. Use this step to finish the work completely – use a metal kidney to polish the surface, or a slightly damp sponge to give a freshly thrown look. Wipe the sponge around the rim after trimming, and check the inside of the pot for dags! Trimming slip cast work: Usually I will trim the rims of your work on the wheel the following day to make that stage easier, however you will still need to check your work for lumps and bumps. Last but not least – check that your name is still clearly on the bottom of your work. Step Five – Bisque Firing When the work is completely dry it can go into the bisque kiln. The bisque kiln is fired to 1000°C. This process burns off the water in the clay as well as some of the chemically bound water. The structure of the clay is not altered that much at this temperature. Inside the bisque kiln, the work is stacked a little, small bowl inside a larger bowl and onto a heavy plate. Smaller items like decorations or drink coasters might get stacked several high. Consideration is paid to the weight of the stack and shape of the work. A bisque kiln can fire about one and a half times the amount of work that the glaze kiln can fire. The firing takes about 10 hours to complete the cycle and about two days to cool down. Once it has been emptied the work is placed in the glaze room ready for you to decorate! Step Six – Glazing Decorating your work with colour can be a lot of fun – and time consuming! There are three main options for surface treatment at this stage: Oxide Washes Underglazes Glazes Washes and underglazes do not “glaze” the work – It will still need a layer of glaze to fully seal the clay (washes don’t need glaze on surfaces not designed for food or liquid as they can gloss up a little on their own). Underglazes are stable colourants that turn out pretty much how they look in the jar. They can be mixed with each other to form other colours and can be used like water colours to paint onto your work. Mostly they should have a clear glaze on top to seal them. Oxides are a different species – the pink oxide (cobalt) wash turns out bright blue for instance. They don’t always need a glaze on top, and some glazes can change the colour of the wash! The glazes need no other “glaze” on top! Be careful of unknown glaze interactions – you can put any combination of glaze in a bowl or on a plate, but only a single glaze on the outside of any vertical surface! Glazes are a chemical reaction under heat. We don’t know the exact chemicals in the Mayco glazes we use. I can guess by the way they interact with each other, however, on the whole, you need to test every idea you have, and not run the test on a vertical surface! Simply put, glaze is a layer of glass like substance that bonds with the clay underneath. Clay is made of silica, alumina and water. Glaze is made of mostly silica. Silica has a melting point of 1700°C and we fire to 1240°C. The silica requires a “flux” to help it melt at the lower temperature. Fluxes can be all sorts of chemicals – a common one is calcium – calcium has a melting point of 2500°C, however, together they both melt at a much lower temperature! Colourants are metal oxides like cobalt (blue), chrome (green through black), copper (green, blue, even red!), manganese (black, purple and pink) iron (red brown), etc. Different chemicals in the glaze can have dramatic effects. for example, barium carbonate (which we don’t use) turns manganese bright pink! Other elements can turn manganese dioxide brown, blue, purple and reddish brown. Manganese dioxide is a flux in and of itself as well. So, glazes that get their black and purple colours, often interact with other glazes and RUN! Our mirror black is a good example – it mixes really well with many glazes because it fluxes them – causes them to melt faster. It will also bring out many beautiful colours in the glazes because it’s black colouring most definitely comes from manganese dioxide! Glaze chemistry is a whole subject on it’s own! We use commercial Mayco glazes on purpose – for their huge range of colour possibilities, stability, cool interactions, artistic freedom with the ability to easily brush the glazes on and ease of use. We currently have almost 50 glazes on hand! A major project is to test the interactions of all glazes with each other. That is 2,500 test tiles!!!! I’m going to make the wall behind the wheels the feature wall of pretty colours! Step Seven – Glaze (Gloss or sometimes called “Glost”) Firing Most of the time this is the final stage of making your creation (but not always!) The glaze kiln goes to 1240°C. This is called cone 6, or midrange. It is the low end of stoneware temperatures. Stoneware clays and glazes are typically fired at cone 8 – 10, that is 1260 – 1290°C. The energy requirement to go from 1240°C to 1280°C is almost a 30% more! Our clay is formulated to vitrify (mature, turn “glass-like”) at 1240°, as are our glazes. A glaze kiln take around 12 hours to reach temperature and two to three days to cool down. Sometimes a third firing process is required – this is for decoration that is added to work after the glaze firing. For example – adding precious metals and lustres. this firing temperature is usually around 600 – 800°C depending upon the techniques being used. There are many students interested in gold and silver trims – we will be doing this third type of firing soon! After firing your work will be in the student finished work shelves. Remember to pay for it before you head out the door! There is a small extra charge for using porcelain clay (it’s more than twice the price of regular clay), and for any third firing process! Once your work has been fired it can not turn back into clay for millennia – so don’t fire it if you don’t like it! Put it in the bucket for recycling. https://firebirdstudios.com.au/the-process-of-making-pottery/ This bowl is an example of kitchenware used in the 19th century and still in use today.Bowl white ceramic plain that has two sets of edging around lip. Inside bowl has plaster designed to look like cooking mixture.flagstaff hill, warrnambool, shipwrecked-coast, flagstaff-hill, flagstaff-hill-maritime-museum, maritime-museum, shipwreck-coast, flagstaff-hill-maritime-village, kitchen equipment, ceramic -
 Flagstaff Hill Maritime Museum and Village
Flagstaff Hill Maritime Museum and VillageDomestic object - Bowl, J & G Meakin, Late 19th or early 20th Century
The Process of Making Pottery Decorating, Firing, Glazing, Making, Technical There is a rhythm and flow to clay. It can’t be done all at once! Even the making process! It can take weeks to get everything done, especially if you can only work on your pottery once a week! Even though we have three hour classes, it’s often just not enough time! Here is an overview of some of the processes so you have a bit more grasp on some of the technical stuff! Step One – Design There are SO many ideas out there for making stuff in clay! From delicate porcelain jewellery, through to heavy sculptural work and everything in between. Deciding your direction is sometimes not that easy – when you first start, try everything, you will naturally gravitate to the style that you enjoy! The options and variations are endless and can get a wee bit overwhelming too! Check in with me before you start to ensure your ideas will work, what order you might do things, how you could achieve the look you are seeking and any other technical data required! Step Two – Making Clay is thixotropic. This means that as you work with it, the clay first gets sloppier and wetter, before is begins to dry in the atmosphere. For most things, you simply can’t do all parts of the project at once. An example of work order might look like: Get last weeks work out from the shelves Prepare clay for today’s work – roll your clay, prepare balls for throwing, make the first stage of a pinch pot) Clean up last week’s work and put it on the shelf for bisque firing Check that you have any glazing to do – and do enough of it that you will have time to finish your main project Do the next step of your next project – there might be a further step that can’t be complete immediately, in that case, wrap your work well and put onto the shelves. Letting your work rest for a while can really help keep your work clean and professional looking. Many things require bagging under plastic to keep it ready for work the next week – put your name on the outside of the bag so you can find your work easily. We have stickers and markers. Consider how you want to decorate your work – coloured slip can be applied at a fairly wet stage (remembering that it will make your work even wetter!). Trying to apply slip to dry clay won’t work! If you want to do sgraffito – you will need to keep the work leather hard (a state of dryness where you can still work the clay with a little effort and a little water and care). Step Three – Drying Most of the time your work can go into the rack uncovered to let it dry out for the following week. If you want to continue forming or shaping you will need to double bag your work – put your work on a suitable sized bat and put the bat in a bag so the base of the bag is under the bat, then put another bag over the top of the work and tuck the top of the bag under the bat. If you want to trim (or turn) your thrown work the following week, it should also be double bagged. If your work is large, delicate, or of uneven thicknesses, you should lightly cover your work for drying. When considering the drying process, bare in mind the weather, humidity and wind! The hotter and dryer, the faster things dry and work can dry unevenly in the shelves – this can lead to cracking – another time to lightly cover your work for drying. Step Four – Trimming and Cleaning Up Your work is dry! It is called greenware now and it is at it’s most fragile! Handle everything with two hands. I often refer to soft hands – keep everything gentle and with your fingers spread as much as possible. Try to not pick up things like plates too much, and always with both hands! Before your work can be bisque fired it should be “cleaned up”. You work won’t go into the kiln if it has sharp edges – when glazed, sharp edges turn into razor blades! Use a piece of fly wire to rub the work all over – this will scratch a little so be light handed. Use a knife or metal kidney to scrape any areas that require a bit more dynamic treatment than the fly wire offers! Finally, a very light wipe over with a slightly damp sponge can help soften and soothe all of your edges and dags! Trimming thrown work: If you are planning to trim (or turn) your thrown work (and you should be), make sure you bag it well – your work should be leather hard to almost dry for easiest trimming. Use this step to finish the work completely – use a metal kidney to polish the surface, or a slightly damp sponge to give a freshly thrown look. Wipe the sponge around the rim after trimming, and check the inside of the pot for dags! Trimming slip cast work: Usually I will trim the rims of your work on the wheel the following day to make that stage easier, however you will still need to check your work for lumps and bumps. Last but not least – check that your name is still clearly on the bottom of your work. Step Five – Bisque Firing When the work is completely dry it can go into the bisque kiln. The bisque kiln is fired to 1000°C. This process burns off the water in the clay as well as some of the chemically bound water. The structure of the clay is not altered that much at this temperature. Inside the bisque kiln, the work is stacked a little, small bowl inside a larger bowl and onto a heavy plate. Smaller items like decorations or drink coasters might get stacked several high. Consideration is paid to the weight of the stack and shape of the work. A bisque kiln can fire about one and a half times the amount of work that the glaze kiln can fire. The firing takes about 10 hours to complete the cycle and about two days to cool down. Once it has been emptied the work is placed in the glaze room ready for you to decorate! Step Six – Glazing Decorating your work with colour can be a lot of fun – and time consuming! There are three main options for surface treatment at this stage: Oxide Washes Underglazes Glazes Washes and underglazes do not “glaze” the work – It will still need a layer of glaze to fully seal the clay (washes don’t need glaze on surfaces not designed for food or liquid as they can gloss up a little on their own). Underglazes are stable colourants that turn out pretty much how they look in the jar. They can be mixed with each other to form other colours and can be used like water colours to paint onto your work. Mostly they should have a clear glaze on top to seal them. Oxides are a different species – the pink oxide (cobalt) wash turns out bright blue for instance. They don’t always need a glaze on top, and some glazes can change the colour of the wash! The glazes need no other “glaze” on top! Be careful of unknown glaze interactions – you can put any combination of glaze in a bowl or on a plate, but only a single glaze on the outside of any vertical surface! Glazes are a chemical reaction under heat. We don’t know the exact chemicals in the Mayco glazes we use. I can guess by the way they interact with each other, however, on the whole, you need to test every idea you have, and not run the test on a vertical surface! Simply put, glaze is a layer of glass like substance that bonds with the clay underneath. Clay is made of silica, alumina and water. Glaze is made of mostly silica. Silica has a melting point of 1700°C and we fire to 1240°C. The silica requires a “flux” to help it melt at the lower temperature. Fluxes can be all sorts of chemicals – a common one is calcium – calcium has a melting point of 2500°C, however, together they both melt at a much lower temperature! Colourants are metal oxides like cobalt (blue), chrome (green through black), copper (green, blue, even red!), manganese (black, purple and pink) iron (red brown), etc. Different chemicals in the glaze can have dramatic effects. for example, barium carbonate (which we don’t use) turns manganese bright pink! Other elements can turn manganese dioxide brown, blue, purple and reddish brown. Manganese dioxide is a flux in and of itself as well. So, glazes that get their black and purple colours, often interact with other glazes and RUN! Our mirror black is a good example – it mixes really well with many glazes because it fluxes them – causes them to melt faster. It will also bring out many beautiful colours in the glazes because it’s black colouring most definitely comes from manganese dioxide! Glaze chemistry is a whole subject on it’s own! We use commercial Mayco glazes on purpose – for their huge range of colour possibilities, stability, cool interactions, artistic freedom with the ability to easily brush the glazes on and ease of use. We currently have almost 50 glazes on hand! A major project is to test the interactions of all glazes with each other. That is 2,500 test tiles!!!! I’m going to make the wall behind the wheels the feature wall of pretty colours! Step Seven – Glaze (Gloss or sometimes called “Glost”) Firing Most of the time this is the final stage of making your creation (but not always!) The glaze kiln goes to 1240°C. This is called cone 6, or midrange. It is the low end of stoneware temperatures. Stoneware clays and glazes are typically fired at cone 8 – 10, that is 1260 – 1290°C. The energy requirement to go from 1240°C to 1280°C is almost a 30% more! Our clay is formulated to vitrify (mature, turn “glass-like”) at 1240°, as are our glazes. A glaze kiln take around 12 hours to reach temperature and two to three days to cool down. Sometimes a third firing process is required – this is for decoration that is added to work after the glaze firing. For example – adding precious metals and lustres. this firing temperature is usually around 600 – 800°C depending upon the techniques being used. There are many students interested in gold and silver trims – we will be doing this third type of firing soon! After firing your work will be in the student finished work shelves. Remember to pay for it before you head out the door! There is a small extra charge for using porcelain clay (it’s more than twice the price of regular clay), and for any third firing process! Once your work has been fired it can not turn back into clay for millennia – so don’t fire it if you don’t like it! Put it in the bucket for recycling. https://firebirdstudios.com.au/the-process-of-making-pottery/This bowl was made by renowned pottery company J & G Meakin of England. The firm was established in the mid-1800's. The bowl is an example of kitchenware used in the 19th century and still in use today.Bowl; white ceramic, round and tapering inwards towards base. Made by J and G Meakin England.On base, 'Ironstone China Reg SOL 391413' with symbolflagstaff hill, flagstaff hill maritime museum and village, warrnambool, maritime museum, maritime village, great ocean road, shipwreck coast, mixing bowl, food preparation, j & g meakin, pottery, stoke-on-trent, kitchen equipment, ceramic -
 Federation University Historical Collection
Federation University Historical CollectionComputer, Mutlitech Industrial Corporation, Micro-Professor MPF-IP and manuals, 1983 (estimated)
The Micro-Professor I Plus (MPF-IP) was a low cost, versatile microcomputer system featuring sophisticated software and hardware capabilities. (MPF-IP) boasted a display panel with the ability to display 20 characters using 16-segment fonts. All 64 standard ASCII characters could be displayed. The operation of the MPF-IP was controlled by an 8k monitor program which resides in the Read Only Memory (ROM). The monitor, aided by 4k Random Access Memory (RAM), enabled the user to enter a comprehensive set of single keystroke commands, making it easier for the user to use the CPU, memory and I/0 devices. This allowed the user to concentrate of microprocessor software development and application design. The system allowed printing at 48 lines per minute, and the ability to permanently record the commands, data, programs, status and other messaged. Each character printed by the printer is in a 5 by 7 dot matrix. Although the prime purpose of the programming was for machine language object code formed as hexadecimal numbers, the Micro-Professor has an embedded Tiny Basic interpreter for which formation of some of the alpha characters using a standard 7 segment display was ingenious. The program memory consisted of non volatile 2 kilobytes electrically programmable ROM whilst the Random Access Memory came with 2 kilobytes of static RAM but could be upgraded to 4 kilobytes by insertion of another chip. The entire memory space of 64 kilobytes was accessible by way of the terminals on the left hand side of the board. Engineering and Science students from the Ballarat School of Mines and the Ballarat College of Advanced Education used a class set (as they were relatively inexpensive at approx. $100 each) during the mid to late 1980s. Student were encouraged to borrow the Micro-Professors in order to assist in learning how to use them. Only one was ever not returned on time. When pressed to return the device the student confessed that his dog had chewed the plastic case. This is still in our collection complete with bite marks! The Micro-Professor used a Zilog Z80 microprocessor. This was the most powerful of the 8 bit microprocessors at the time. Zilog was derived from the Intel 8080 microprocessor. The Z80 had 158 instructions of which the Intel 78 instructions were a subset. The Intel processor continued on through development in the IBM computers as 8086, 80286, 80386, 80486 and later the pentiums. Zilog lost most of its market share when it developed the 16 bit Z8000 microprocessor. Although the microprocessor was excellent, the lack of peripherals caused users to abandon Zilog products. A brown and gold plastic box containing a microcomputer for use in classrooms. Four manuals are titled 'Micro-Professor MPF-IP user's Manual', 'MPF-I Experiment Manual (Software/Hardware)', Micro-professor MPF-IP experiment Manual (Software/Hardware)' and Micro-Professor MPF-I Monitor Program Source Listing.microcomputer, micro computer, micro professor, electronics -
 Federation University Historical Collection
Federation University Historical CollectionBook, Machinery for Metalliferous Mines, 1894, 1894
The 1st edition of this famous work, giving an excellent account of the machinery used in late 19th century metal mining in the UK and overseas is very rare. It covers a wide range of equipment - pumps, steam engines, drills, winding engines, stamps & concentration mills, aerial ropeways, tramways and early uses of electricity etc. Brown hard cloth covered book. xvi 564 pages with additional advertisements, with over 300 illustrations and drawings, some fold out. Chapters include Water as a motive power, Wind engines and ventilating machinery, Steam boilers/engines and oil engines, hoisting machinery, draining of Mines, pumping engines, rock drilling machinery, boring machinery, concentration machinery, sizing and classifications trommels, joggers and jigging, fine concentration, milling of gold ores, milling of silver ores, amalgamation plates and machinery, dry and roasting machinery, chlorination and cyandide processes for the extraction of gold, electricity as a motive power for mining, electric lighting and blasting, aerial wire ropeways, transport by rail and road. There a a number of lovely line illustrations in the book including: Poncelot's undershot waterwheel; Fromont furnace;Victor turbine; Pelton waterwheel; Root's positive blower;Cross section and front elevation of Lancashire boiler; Robey's Compound Mill Engine; Portable Winding Plant; Iron Pit Head Gear ; Loading Arrangement in an Incline Shaft; kibble; Worthington Pump; California Pump; Scram's Air Compressor; Rock drill Bits; Special Sharpening tools; Boring tools;Rotating Picking table; Ore Feeder; roller crusher; stamp battery; round buddle; slime table; vanner; amalgamating plant; belt elevator;roasting furnace;splicing wire rope; capel; tipping waggon;mining, cornish pump, linkenbach table, water wheel, ventilation, oil engine, california, america, water, steam boilers, steam engines, oil engines, pumpimg, rock drilling, boring, jiggers, milling, silver, gold, drying and roasting, chlorination, cyaniding, lead, zinc, copper, electricity, electric lighting, wire ropes, transport, wind engine, poppet head -
 Federation University Historical Collection
Federation University Historical CollectionBook, Cyaniding for Gold, 1939, 1939
500 of these books were printed, and many were used in the field, making good copies very rare. A simple but detailed account of the process written especially for the working miner and the small syndicate, the Quintessential Australian gold Mining book.Orange, hard, cloth covered b270 page book with brown dust jacket. Chapters include sampling sand and slime dumps; treating sand; aeration; treatment of slime; smelting; solution testing; cyanide solution; air- slaked lime; cyanding in Queensland; copper troubles; treatment of concentrates; amalgamations; assaying; floatation; chemistry; testing for minerals. Includes photographic reproductions of a cyanide plant for sand; aeration tower; mixer tank; Eleanora cyaniding plant; mining, gold, cyanide, cyaniding, leaching, smelting, slimes -
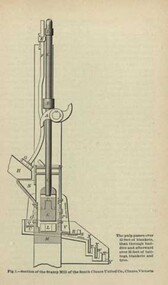 Federation University Historical Collection
Federation University Historical CollectionBook, The Scientific Publishing Co, Stamp Milling of Gold Ores, 1897
Brown, hard, cloth covered book of 260 pages, including an index and illustrations. The contents include the Philosophy of the Stamp-Milling Process; Gilpin county colarado; Typical Stamp Mills of California; Milling in Black Hills South Dakota; Early Australian methods, more modern Australian Methods; Gold Milling at Bendigo; Double discharge Mortars in Victoria; Stamp Mills of Otago New Zealand, Review of Australian Practice, Wear and Tear of a Mill; Flouring of Mercury. Illustrations include South Clunes United Company, Crushing Mill at Ballarat, Battery at Bendigo.mining, milling, colorado, ballarat, bendigo, california, clunes, america, united states of america, modern australian methods, star of the east, sebastopol, britannia united, bakery hill, north cornish mill, daylesford, new normanby, north cornish -
 Federation University Historical Collection
Federation University Historical CollectionBook, Crosby Lockwood and Son, The Metallurgy of Gold, 1896, 1896
... mining in California, Gold as a metal; Hydraulic Process... Process; treatment of Gold bearing Ores; Crushing and Amalgamation ...M. Eissler A.I.M.E. was a member of the Institute of Mining and Metallurgy.Red Hardcovered book of 678 pages with around 250 illustrations and numerous folding plates. Contents include: gold mining in California, Gold as a metal; Hydraulic Process; treatment of Gold bearing Ores; Crushing and Amalgamation (Queensland, California); Mills; Buddle; concentrator; Roasting ores; Roasting Pyritic Ores; Hydro-Metallurgy; Chlorination; Electro-Metallurgy; Cyanide; Erection of a Cyanide Plant; Chemistry of the Cyanide Process; Spelting, CupulationCrushing ore; 160 stamp battery; Mill site and battery; causes of failure in gold mining, South African Gold Fields; Gold in Australia (pg 589) Advertisements include: Fraser & Chalmers; Bowes scott & Western Ltd; Fried. Krupp Grusonwerk; Stanley; Humbolt Engineering Works Co., Townson & Mercer.gold, mining, metallurgy, pyrites, cupel, brook, rand, california, america, gold mining -
 Federation University Historical Collection
Federation University Historical CollectionBook, The Cyanide Process of Gold Extraction, 1906, 1906
... The Cyanide Process of Gold Extraction, 1906... of America, Mexico, India, Canada. The Cyanide Process of Gold ...Maroon hard covered book of 239 pages. Along with technical information the book looks at cyanide processing around the world including South Africa, New Zealand, Kalgoorlie, Golden Horseshoe Mine, Great Boulder Main Reef, New South Wales, United States of America, Mexico, India, Canada.cyanide, mining, mcarthur-forrest process, laboratory, slimes, leaching, zinc precipitation, siemens-halske process, cyanide plantbutters' distributer, cyanide poisoning antidotes, south africa, new zealand, waihi mine, waikino, south kalgoorlie, united states, mexico, india -
 Federation University Historical Collection
Federation University Historical CollectionCertificate, Ballarat School of Mines, William Corbould's Ballarat School of Mines Metallurgy Certificate, 11/07/1883
William Corbould was the son of a Ballarat tailor. He attended Ballarat College, and obtained certificates in assaying and metallurgy at the Ballarat School of Mines (SMB) in 1883, studying under the revered Professor Mica Smith. Corbould was not a born student and remembered his first experience at SMB: 'From the Registrar's Office I was led to be introduced to the Professor of Chemistry, one Mica Smith. The initial encounter gave me little encouragement - his large laboratory was filled with hundreds of bottles bearing strange labels with queer symbols on them. My heart sank. At the first opportunity I grabbed my hat and made for the door, but the good professor called me back. I pointed out that I was never any good at school ... so it was no use pretending to be clever enough to understand all those weird symbols! The Professor told me not to worry about that and took me to one of the benches where he found a blowpipe and a charcoal block. Mixing together two powders from bottles on the shelf he transferred a sample to the charcoal and directed the bunsen flame onto it. Soon it began to melt and a white bead appeared in front of my eyes. He then took a test tube and added a little colourless liquid from each of two bottles. A beautiful dark blue colour appeared. My interest was won.' During Corbould's mining career he travelled to Europe twice, and visited most of Australia's main mining fields. Corbould started his career as an assayer at Pinnacle Silver Mine, Silverton, and was then a self-employed assayer at Broken Hill. Corbould became an assayer for the infant BHP mine, and later worked in Kalgoorlie and Coolgardie, including managing Hannan's Reward, the oldest gold mine on the Kalgoorlie gold field. He spent 13 years at the Mount Elliott copper fields as general manager. In 1923, at the age of 57, Corbould went to Mount Isa and reported on options, experimented with new metallurgical processes and floated a company. John Carden of CRA said: 'Corbould was the man who brought Urquhart to Mount Isa. He was the man who made it all possible. He is tremendously important in the Mount Isa story, because he was the first technical man, the first professional man on the scene. He was responsible as I said, for bringing finance to the place, but I think even more importantly he was the first man to recognise the need to put all the little claims in the Mount Isa discovery together. I think perhaps his major contribution to Mount Isa was this amalgamation on the various claims. He recognised that the ore bodies at Mount Isa were not as rich as Broken Hill and they would never have survived had it been fragmented, so he was terribly important.' After completing major financial negotiations for Mt Isa Mine from London in 1927 Corbould remained in Europe where he remained until his death. Corbould was awarded the Legion of Honour of the American Institute of Mining and Metallurigical Engineers for fifty years service. Corbould died at Monaco in 1949 at the age of 82. (http://guerin.ballarat.edu.au/curator/honour-roll/honourroll_Corbould,William.shtml)A white paper certificate with black printed and handwritten text, and a blue Ballarat School of Mines seal. The certificate is signed by Andrew Berry (Registrar) and James Oddie (Vice-President).Signed on the left 'W.H. Corbould'mining, ballarat school of mines, mining alumni, metallurgy, james oddie, andrew berry, william corbould, corbould, berry, oddie -
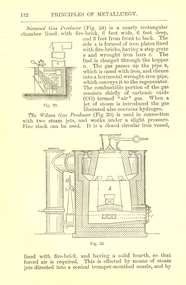 Federation University Historical Collection
Federation University Historical CollectionBook, Principles of Metallurgy, 1901, 1901
Maroon hard covered book of 388 pages. The book was written for the budding metallurgist, constituting an elementary treatise on the subject, dealing with principles rather than processes, the contents include: Intro., Definition, Properties, Principles, Alloys, Slags, Fuel, Iron, Steel, Silver-Gold-Platinum, Copper-Zinc, Lead-Tin, Nickel-Cobalt, Aluminium, Mercury, Antimony-Arsenic, Bismuth, Index.metallurgy, brook, hiorns, pig iron, steel, silver, acid, stamp battery, ores, zinc, copper, antimony -
 Federation University Historical Collection
Federation University Historical CollectionBook, Ballarat School of Mines Students' Magazine, 1902-1906, 1902-1906
The Ballarat School of Mines is a predecessor institution of Federation University Ausgtralia.Five copies of the Ballarat School of Mines Students' Magazine bound in a blue hard cover and quart bound in calf. 1902 - Retirement of Herbert L. Krause 1905 - Mt Lyall Ore Deposits Images: Ballarat School of Mines Sports Association Committee and Officers, 1905 Standing left to right: W. Pearce, T. Williams, S. Hepburn, Seward, W. Figgis, J.R. Reid, P. Elliott Sitting left to right: P.A. Pratt, N. Buley, John Sutherland, H.R Murphy, J. Inglis, R. Nevett 1906 Excursion to Broken Hill, The Artistic Printing Process, Hints on Portraiture, Arizona, Cornish Mining Images Ballarat School of Mines and AusImm at the Central Mine, Broken Hill; At Iron Knob, Brown Boveri Turbo Generator at Block 10, Junction Mine plant or Potter Process, Broken Hill Propriety Mill, Carlyon's Hotel, Sam Jamieson, Electrical Labortory, Ballarat School of Mines Rowing Eight, Sports Committee,ballarat school of mines, magazines, sports committee, thomas williams, mount lyell, w. pearce, t. williams, s. hepburn, seward, w. figgis, j.r. reid, p. elliott, p.a. pratt, n. buley, john sutherland, h.r murphy, j. inglis, r. nevett, herbert l. krause, broken hill, artistic printing process, hints on portraiture, arizona, cornish mining, ausimm at the central mine, broken hill, iron knob, brown boveri turbo generator at block 10, junction mine plant or potter process, broken hill propriety mill, carlyon's hotel, sam jamieson, electrical labortory, ballarat school of mines rowing eight, sports committee,, rowing on lake wendouree, boat shed, h. valentine, l. seward, c. macgennis, d. don, m. gaunt, h. owne, l. nott, s. leathes, j.a. reid, h.r. murphy, alfred mica smith, r. hosking, zeehan, moonta, wallaroo, smelting silver, south africa, south australia excursion, sunnyside woollen mills, tasmanian excursion, swedish iron, mt jukes excurson, western australian gold ore, wynne-grant furnace, bhp sintering slimes, leggo furnace, mine ventilation, mount morgan mine, mount pani ltd, new zealand coal, adelaide school of mines, students' association, at iron knob, ausimm, excursions, visits, south australian excursion -
 Federation University Historical Collection
Federation University Historical CollectionBook, Ballarat School of Mines Students' Magazine, 1907-1909, 1907-9
Bound volume of the Ballarat School of Mines Students' Magazine for 1907-1909. Articles include the location and pegging of a tramway Route, the transmission of Power by Rope Driving by A.E.C. Kerr, A visit to the Briseis Mine, and New Brothers' Home, Mining Engineers in Tasmania, Obituaries for David Ham and Matthew Lyndsay, Pioneer Mining at Leichhardt, Automatic Ore Feeders and Chacedony Park by J.H. Adams. Images include the Ballarat School of Mines Football Team and the Ballarat School of Mines Sports Committee. 1907 * Hubert Krause 1908 * The location and Pegging of a Tramway Route by Yamba * The Unity of things by John Brittain * The transmission of power by Rope Driving, by A.E.C. Kerr * A visit to the Briseis Mine, and New Brothers' Home (includes photographs) * Mining Engineers in Tasmania * Limericks Sluiced at Snake Valley * David Ham Obituary * Matthew Lyndsay Obituary * Some Bunsen Memories by Professor Alfred Mica Smith * Geological Camp to Daylesford * Practical Mathematics by Hubert F. Hall * Pioneer Mining - Leickhardt * Automatic Ore Feeders * A Unique Ore Deposit (Lucknow Goldfield, NSW) * A country Ramble * The Alkaline Titration for Zinc * Chalcedony Park, Arizona, United States of America by J.H. Adams * Notetaking at Lectures * Smelter Cost-Keeping by Oliver E. Jaeger * Benefits of Physical Culture * Mineral Tasmania and its Wonderful Resources by F.F. Bradford * A holiday Trip to Queensland * The Compression of Air as Applied to Mining * Machine V Hand Drilling * The mining in the Malay States * William Thomas Grownow Obituary (includes image) * Timbering Stopes (Includes images) * Trip to Melbourne * Two Problems of Alluvial Mining by Richard Hain * The Berry Leads (includes plan of mines) * Black Pudding Supper * Observations for Meridian * NOtes on Coking Plants (includes plan) * How Old is the Earth * Conglomerations * A Pat Formula * The making of a Suitable Muffle for General Assay Work * Reduction of Gold Chloride by Charcoal * Chemical Definitions * Old Boys (R.J. Allen, W.B. Blyth, F. Brinsden, Lindsay D. Cameron, George W. Cornwell, John M. Currie, C.M. Harris, T. Wighton-Hood, W. Lakeland, W.S. Macartney, Fred A. Marriott, Karl B. Moore, G.E. Sander, Sherb. H. Sheppard, Norman S. Stuckby, John Sutherland, Lewis A. Westcott, Walter White, O.C. Witherden, Gerald Young) 1909 * The Graduates Problem * An Interesting Nevada Cyanide Plant (Bamberger De Lamar Gold Mines) * Easter Geological Camp to Ingliston * New Methods for the Volumetric Estimation of Lead * William Charles Kernott Obituary * Mount Morgan Mine by G.W. Williams * Tin Dredging at Tingha, New South Wales * Notes on the Iodide estimation of Copper * Photograph of the Ballarat School of Mines Students' Association Committee * The Importance of Mine Ventilation to the Students by F. Howells * Life in Mining Camp * Wise Words to Mining School Students * Military Search Lights * Gleaning s on resistance * Ministerial Visit to the Ballarat School of Mines by the Hon. A.A. Billson, Minister of Education , accompanied by Frank Tate, Director of Education. * Picture Making in Photography Composition * Mountains of Fire * A Walking Engine - New Military Machine - Guns into Action - Caterpillar No. 1. (Tank) * Fireless Locomotives * Elmore Concentrating Process (includes plan of Elmore Concentrator) * Mount Pani Limited * Surveying for Irrigation * Weights and Measures * Carbon Monoxide * Joseph Francis Usher obituary * The Problem of the Metalliferous Veins * Ballarat School of Mines Ambulance Class * Treatment of Gold in a battery Without the Use of Copper Plates by E.C. Hurdsfield * Meteorites * Werribee Gorge * Dredging in the Ovens Valley * Electrical Chatter - Electrocution by an Imaginary Eye-Witness * The Electric Furnace in Iron Metallurgy ballarat school of mines, ballarat school of mines football team, football, sports, tramway, mining engineers, david ham, matthew lyndsay, jack adams, berry lead, hepburn consuls, madame berry, west ristori, ristori no 1, allendale, dyke's co, charleson's mill, australian extended, mining, r.j. allen,, w.b. blyth, f. brinsden, lindsay d. cameron, george w. cornwell, john m. currie, c.m. harris, t. wighton-hood, w. lakeland, w.s. macartney, fred a. marriott, karl b. moore, g.e. sander, sherb. h. sheppard, norman s. stuckby, john sutherland, lewis a. westcott, walter white, o.c. witherden, gerald young, ballarat school of mines students' association, hubert krause, krause, vfl, afl -
 Federation University Historical Collection
Federation University Historical CollectionMagazine, Ballarat School of Mines Students' Magazine, 1898-1901, 1898-1901
Bound copies of the Ballarat School of Mines Students' Magazine, 1898-1901 Vol 1, No. 1, September 1898 * News and Notes (Ballarat School of Mines Museum, J.F. Usher, New British Pharmacopoeia, excursion to Bendigo) * History of the Ballarat School of Mines * Current Topics (Federation, Gladstone, Anglo-American Alliance) * Of Custom * Discovery of Coolgardie * Mining Notes(Clunes, Pitfield, Birthday Mine, Western Australia, Transvaal, Mt Bischoff, Rand Drill Co.) * From the Journals * The Societies - (Student Association, Ballarat Field Club and Science Society, Ballarat Photographic Club) * Original Poetry * Sports * Students' Association Committee Meetings * On the Increase of Temperature of the Earth With Increased Depth Vol 1, No. 2, October 1898 * Notes about some of the Past Students (E.M. Weston, J.A. Porter, H.R. Sleeman, G.E. Sander, B.C.T. Solley, T. Rhys, C. Burbury, D. McDougal, J. Matsen) * Excursion to Daylesford, p.3 * History of the Ballarat School of Mines (continued) * The Soudan * Greater Melbourne * Image of J. Hopkinson, electrical engineer killed ascending the Alps * What is Science * Mining Notes (Pitfield Plains, Victoria United G.M.Co., Lithgow, Avoca, great Cobar, Mt Whycheproof) * Student's Association (women's franchise) * Sports Vol 2, No. 1, March 1899 * News and Notes * History of the Ballarat School of Mines (continued) * Notes of Victorian Geology, 1. Granites, by Thomas S. Hart * Sir William Crookes * Summaries and Notes from the Mining Journals * Students' Association * Sports * The Bush Assayer * Solubility of Gold-Silver Alloys in Potassium Cyanide * Correspondence Vol 2, No. 2, April 1899 * News and Notes (Smythesdale Excursion, New Buildings, A.S. Coyte, R.J. Allan) * History of the Ballarat School of Mines (Continued) * The New Students (J. Owen, A. Clayton Morrisby, A.S. Atkin, J. Alexander Reid, Alfred G. Johnston, L. Lowe, F.H. Dalton, W.M. Robertson, A. Hacke, H.L. Giles, W. Martin, E. Walshe, H.L. Krause, R. Sawyer) * Berringa by Oh'E Jay * Summaries and Notes from the Mining Journals * Mount Magnet to Victoria - A Long Bicycle Trip * 1898 Examination returns * Sports Vol 2, No. 3, May 1899 * Technical Education and the Proposed Affiliation of the Schools of Mines with the Melbourne University. * Laying of the Foundation Stone of the New Classrooms (now Administration Building). Alexander J. Peacock * News and Notes (Past Students - A.S. Lilburn, J.W. Sutherland, J. Richardson, E. Prendergast, J. Wallace, J. Kidd, J. Lake, Mathew Thompson), Coolgardie Exhibition. * Trip to Lal Lal * Students' Association * Summaries and Notes from the Mining Journals * Professor Henry Louis on Mining Education * Corrections Used in Chaining by C.W. Adams * The Black Horse Cyanide Plant * Sports * Completed List of 1898 Examinations Vol 2, No. 4, June 1899 * News and Notes * The Education Problem by D.N. McLean * A Few Hints on Histological Technique by Emil Gutheil * Summaries and Notes from the Mining Journals * Students' Association * A Visit to the Skipton Caves (Mount Widdern, Ormand Hill, volcano, Emu Creek, Mount Kinross, Mount Elephant, Mount Vite Vite, Mount Kinross, Mount Hamiston) * Mount Magnet To Victoria (cont) * The New Engines at the Ballarat Woollen Mills - includes image of the Compound 700 H.P. Engines constructed for the Ballarat Woollen Mills by Austral Otis Company and consulting engineers Monash and Anderson. * Sports * Original Poetry * Correspondence Vol 2, No. 5, July 1899 * News and Notes (E. Byron Moore, Visit to Britannia Gold Mine, J. Bryant, Visit to Last Chance Mine) * A Few Hints on Histological Technique (cont) by Emil Gutheil * Summaries and Notes from the Mining Journals * Professor Alfred Mica Smith (includes image) * Notes on Victorian Geology Part 2 The Trappean Rocks, by Thomas Hart * Origin of Diamonds * Hydraulic Mining by A.E.C. Kerr * Volcanoes by F.G. Bonney * Analytical Chemistry Notes by Daniel Walker * Some Things Out To Do * Sports * Correspondence Vol 2, No. 6, August 1899 *Summaries and notes from the Mining Journals * Some Regulations of the Academy of Mines at Freiberg * A visit to Mt Lyell Smelters * Professor Gilbert J. Dawbarn (includes image) * Air compressor and Transmission of Power by Compressed air by A.E.C. Kerr * Chemistry Notes by Daniel Walker * Mineralogical Notes, Ballarat by Thomas S. Hart * Kalgurli Gold Mines, W.A. * OUr New Lab Vol 2., No 7, September 1899 * Summaries and Notes from the Mining Journals * Some recent Steam Plants at Bendigo by Gilbert Dawbarn * Professor Thomas Stephen Hart (includes image) * Students Association * Notes on Victorian Geology by Thomas Hart * Centrifugal Pumps * A New Chum's Experience by E.M. Weston Vol 2., No 8, October 1899 * The institute of Chemistry Examinations * A New Method of Qualitative Chemical Analysis by Emil Gutheil * Steam Engine Valves and Valve-Gears by Gilbert Dawbarn * Daniel Walker (includes image) * Notes on Victorian Geology by Thomas Hart * Cyaniding Cripple Creek Tellurides (Metallic Extraction Company) * Notes on Two Ballarat Gravel Pumping Plants, G.A. Wilberforce (Eureka Jennings Co and Yarrowee Sluicing Co) * History of the School of Mines (concluded) Vol 3., No 1, March 1900 * A Journey from Natal to Mashomaland with the British Police * A Plea for Research * New Caledonia by C.A.M. Deane * Notes of Victorian Geology - Lower Palaeoroic Rocks by Thomas Hart * Mt Bischoff Mine and Mill * Summaries and Notes from the Mining Journals * Things we Eat and Drink * Farewell to A.S. Coyte Vol 3., No 1, March 1900 * Mining Education * Model Locomotive made by the apprentices of the Phoenix Foundry, p2 * Glimpses of Rhodesian Police Camp Life * New Caledonia (continued) * Summaries from the Mining and Engineering Journals * Boot and Saddle Vol 3., No 3, May 1900 * A Students' Common Room * Geological Excursion to Hardie's Hill * Notes on Victorian Geology by Thomas Hart * The Planet Venus by John Brittain * Summaries and Notes from the Australian Mining Standard * The Assay Ton * Zeehan Smelters * Electrical Notes by Ohe Jay * Trop of the Cricket Club to Stawell * Students' Association * Solid Hydrogen Vol 3., No 4, June 1900 * The Minister of Mines on Mining Education (Minister A.R. Outtrim) * Lal Lal Geology Trip (Thomas Hart) * Rifle Club now defunct, pg 3 * A Contribution to the Mining Geology of Kalgoorlie, W.A. by Ferdinand Krause (includes cross sections) (Wood's Point, Rand, Johannesburg, South Africa, Gaffney's Creek, Walhalla, Shady Creek, Sago Hill at Cardigan, Bunbury) * Summaries and Notes from the Australian Mining Standard (Buninyong Estate Mine) * Monthly Progress Reports of the Geological Survey * Electrical Notes by John M Sutherland (Telagraphone, phonograph, telephone receiver) * Students' Theatre Party (Gordon Todd, Ohe Jaeger, C.S. Wakley) * Opening of the New Buildings - Ministerial Speeches (Outtrim, W.H. Irvine, New Mining Laboratory, Old Chemistry Building, Battery, Model Mine) * Students' Association * Relief of Mafeking * A Critic Criticised * Things We Eat and Drink by Ohe Jay - Oatmeal, Coffee and Cocoa. Vol 3., No 5, July 1900 * Research * Adelaide Varsity Students at Ballarat * The Manchester-Liverpool Mono Railway * Students Association * *A Contribution to the Mining Geology of Kalgoorlie, W.A. by Ferdinand Krause (continued) (includes cross-sections) * Motive Power, address by Charles A. Parsons * Summaries and Notes from the Australian Mining Standard * Sugar Manufacturing by Sugna * Great Creswick Hydraulic Sluicing Plant (THomas Hart, Ballarat School of Mines Mining Class visit) * Reminiscences of a Students Life in Germany * Football - Ballarat School of Mines v Geelong Grammar School (Australian Rules Football) Vol 3., No 6, August 1900 * Cheap Mine Management * Library * Bendigo School of Mines, pg 3 * Notes on Ore Dressing by T, Vincent, Manager The Zeehan (Tas) Silver-Lead Mines Ltd) * Motive Power * Notes on Broken Hill - Its Mines and Minerals by J. Williams * The Concert * Summaries and Notes from the Australian Mining Standard * The Dandy Duke's Dreadful Demise * The Road Race Vol 3., No 7, September 1900 * Michaelmas Excursion (Melbourne University, Prof Kernot, Applied Mechanics) * Injury to School Property * Return of E. Ditchburn (Boer War) * Mt William Gold-Field visit, pg 3 * The Stoping of Wide Lodes by J.V. Lake (includes cross sections) * Summaries of Notes from the Australian Mining Standard * Notes on Broken Hill Part 2- Its Mines and Minerals by W.J. Williams * Motive Power from the Waves * Electrical Notes * Some Account of Italian Mining (Sarinia, Sicily, Peidmont, Lombardia) by Candido Maglione * Students Association * Should Women Have the Vote by Frank Bessemeres * The School Theatre Parly * Past Students * Poetry * Football * Surveying Rules Vol 3., No 8, October 1900 * Ballarat School of Mines Associateship * An Engineering Laboratory * Students' Practical Work * Notes on Broken Hill Part 3 by W.J. Williams * The Lake View Consols by F.S. Earp - Battery Treatment of Sulpo-Telluride Ore * Neglected Mineral Fields - Eurowie and Warrata * A Glimpse Ahead * News and Notes * A.W. G. McPherson, Boer War * Students Association * Ballarat School of Mines Melbourne Excursion to the Government Electric Lighting Station, Austral-Otis Co, Working Mens College * Ballarat School of Mines Concert in Aid of Soldiers Statue Balance Sheet * Football * Cricket Vol 3., No 8b, November 1900 * Position of the Ballarat School of Mines with Regards to Mining Education * Age Limit * Entrance Examination * Presentation t0 Professor Alfred Mica Smith * Image of a Group of Old Ballarat School of Mines Students in Coolgardie and Kalgoorlie. * Students Association Vol 4., No 1, March 1901 * Espirit De Corps * A few Notes on the Testing of Explosives * Round About Inverell, NSW by F. and J. Mawl * On the Choice of Drawing Instruments * Summaries and Notes From the Technical Journals * Annual Examinations 1900 * New Students * Sporting Notes * The Vale of Coolgardie Mine, Bonnievale, W.A. by G. Stephen Hart * News and Notes (Kerr Grant, C.L. Nash, R. Gordon Todd, Vial) * Editorial Notices Vol 4., No 2, Second Term 1901 * The Metallurgical Treatment of Sulpho-Telluride Ores by L.W. Grayson * Some Metallurgical Difficulties of Aluminium * Diehl's Sulphide Process by A.E. C. Kerr * A Californian Gold Mine by A.E. C. Kerr * New Express Locomotives for the Victorian Government (Phoenix Foundry) * An Excursion to Geelong (Electric Light and Traction Company of Australia) * The Linkenback Table for our New Mining Laboratory (Humboldt Company of Colgne) * Death of Thomas Bath * The Late Alfred G. Johnson (Boer War) * An Introduction to Natural Science by Emil Gutheil * The First Annual School Sports Meeting * Concert in Aid of Magazine Funds * The Men That Made the Concert (C.E. Denniston, W.H. Chandler, Mr White, William Litte Jnr, Marriott, Giles McCracken) * Sports * News and Notes Vol 4., No 2, Third Term 1901 * Bagging-Up - A Sketch * Concentration of Difficult Silver-Lead Ores * Estimation of Chlorine, Bromine and Iodine by D. Runting * Summaries of Notes from teh technical Journals * Notes on the Use and Care of Platinum Ware Common Sense * The Machinery at the Tasmania Gold Mine, Beaconsfield, Tasmania * Mining at Walhalla - The Long Tunnel Mine * Past Students * Mapping our of Agricultural Areas, etc, In Dense Vine Lands, North Queensland by R.A. Suter * News and Notes * Concert Balance Sheet e.m. weston, robert brough smyth, mcdougall, bruce, charles burbury, harrie wood, graham j. hopwood, emil gutheil, daniel walker, thomas hart, thomas stephen hart, m. hacker, schnitzler, f.a., ditchfield, l.h, alfred e.c. kerr, charles harvey, campbell, joseph bryant, campbell & ferguson, gilbert j. dawburn, irving, g.b., kerr, a.e.c., john walter sutherland, william robertson, herbert l. krause, alfred mica smith, binh pham, crosbie, d. jack, ditchburn, j., james hiscock, alfred johnston, reid, j.a., kidd, john, james bonwick, james, j.p, overall, d, e.h salmon, gaynor marquand, williams, w.w., williams, william, deane, c.m., vincent, tom, phillips, g.e., hart, d.w., jarnail suingh, rowlands, e., ferdinand m. krause,, easterby, f.l, parsons, r.g., partington, j.r., vial, s.b., meadows, h, atkins, arthur, john braisted burdekin, w.h. corbould, ditchburn, john, hill, john, otto e. jager, mcpherson, g.t, nicholls, c, thom, j.m., crafter, stewart, john brittain, peter lalor, hardy - commissioner, thomas bath, alf johnston, charles campbell, nash, llewellyn, watson, m.a, gardener, eddie, adamson, s.g, alford, l.c, allen, r.j, arthur, d.w.b., burge, a., willia, cairncross, cooper, i, maurice osric copland, maurice copland, dickinson, s., doepel, dunstan, john, loveday dunstan, eeles, terri, flegeltaub, israel, fletcher, a, fyrar, peter, kerr grant, w.kerr, green, gary, betty harris, harris, c.m., hay, a.l., hearn, hill, martin, james, david, johnston, alfred g, kilner, marion, kingston, thomas, lewin, f.c.k., lilburne, arthur m, linahan, colin, macready, w.h, major birlefco, markwald, henry, mccaffrey, mcfarlane, kaye, mciver, s.k, mellins, b, morton, felicity, w. kenneth moss, ken moss, nash, c.w., nash, neville, nickolls, berkeley, osborne, percy, philp, e., playford, william, reid, e, roberts, gordon, ross, f.c., royce, phillip, sawyer, basil, stewart, r.c., todhunter, i, vaisey, a., vincent, john, vinden, sue, wakley, cecil, watt, james, westcott, lewis, charles w. whyte,, vial, s browning, ballarat school of mines students in coolgardie and kalgoorlie, coolgardie, kalgoorlie, claude maitland, a.l. hay, a.s. lilburne, latham watson, arthur kildahl, thomas copeland, f.a. moss, w.a. hearman, cardoc james, alexander fraser, e.o. watt, g.m. roberts, j.j. dunstan, h.v. moss, j.a. hill,, john dunstan, c.m. harris, william h. corbould, j.w. sutherland, ballarat photographic club, ballarat field naturalists club, ballarat field club and science society, photography, geology, excursions, last chance mine, tasmania gold mine, beaconsfield, tasmania, rand, south africa, mount lyell, ballarat school of mines student excursion to mount lyell, h.l. krause, ferdinand krause, krause, hardie's hill, hardie's hill excursion, lal lal, lal lal excursion, lal lal geology excursion, smythesdale, smythesdale excursion, soudan, south african miners, south star mines, wynne and tregurtha battery, ananconda copper mining, arizona copper mining, boiler plates, british guinea, butte copper smelter, daylesford geology camp, daylesford excursion, diehl process, electric power house ballarat, electric pumps, geelong rope factory, gympie, golden horseshoe estate, c johnstone, jack nichol, c. macgennis, alec saunders, alfred g. johnstone, graeme jolly, william purdie, john mann, maxwell l gaunt, sale school of mines, freiberg school of mines, schools of mines, railway locomotive -
 Federation University Historical Collection
Federation University Historical CollectionDocument - Correspondence, Richard Squire Mining Correspondence
Seems that Tom pays Ned's wages. Numerous bores were sunk , some were opened out and driven in the direction of the reef. Numerous Cross cuts were also made from the main tunnel.Unless otherwise stated the letters are from Dad (Richard Squire) to Tom, Hazel & Kiddies all. .1) Handwritten two page letter from Richard Squire to 'Tom & Hazel & little men' with information regarding the Leigh River Tunnel at Mount Mercer, dated 6/8/28. .2) Handwritten three page letter with information regarding mining operations dated 27.6.29. .3) Handwritten three page letter from Richard Squire to 'Tom & Hazel & Flock' with queries regarding the health of one of their children, other personal information and information regarding mining operations dated 18/7/29. .4) Handwritten one page letter from Richard Squire to 'Tom' dated 20/7/29, regarding the enclosed handwritten receipt with stamp for Call of two shares costing 8 pounds 7 shillings and 2 pence in Leigh River Tunnel Syndicate from Richard Squire dated 13th July 1927. .5) Handwritten two page letter from Richard Squire to 'Tom & Hazel & Pinchers' regarding his health, other personal information and information about the four page report of position of work at 'Leigh River Tunnel' enclosed. The letter is dated 29/11/29 and the report dated Nov 28th 29. .6) Handwritten two page letter with personal information and a suggestion that Tom learn to swim dated 13th Jan 30. .7) Handwritten two page report by Richard Squire regarding Leigh River Tunnel Syndicate plus a one page 'Rough Section' drawing of the tunnel dated 29th April 1930. .8) Typed one page report by Richard Squire regarding Leigh River Tunnel Syndicate and the findings of a new shaft dated 9th December 30. .9) Handwritten one page letter by Richard Squire to 'Tom' regarding mining operations and four handwritten receipts for payment of Calls from Richard Squire and Mr H McLeod and Mr E McLeod with stamps. .10) Handwritten two page letter regarding a meeting he had with the Secretary for Mines, Mr Whitehead dated 22 Feb 1931. .11) Handwritten two page letter regarding the difficulties in financing the mining operation occasioned by the interference of the government department dated 12th Mar 31. .12) Handwritten one page letter regarding the delay in the submission of his letter to the Gold Committee and with details of plans he had sent to Tom separately, dated 1st April 31. .13) Handwritten two page letter by Richard Squire to 'Tom' regarding personal matters including his health and also information regarding Tunnel work, dated May 11th 31. .14) Handwritten two page letter regarding the mine work, how his new employee, Ned, was going and also his health, dated Thur 21st May 31. .15) Handwritten two page letter regarding the Tunnel progress and financial matters dated Tue 26th May. .16) Handwritten three page letter regarding the Tunnel progress dated Thur June 11th. .17) Handwritten two page letter regarding the Tunnel progress, an issue relating to Ned's taxation assessment and his own health, dated 28th June 31. .18) Handwritten three page letter with detailed information on the Tunnel progress and information regarding his health, dated Thur July 23rd. .19) Handwritten two page letter regarding the Tunnel progress and some personal greetings, dated Thur July 30th 31. .20) Handwritten two page letter regarding the Tunnel progress, an agreement with Messrs Read & Peers? and a renaming of a Prospect as Lawaluk instead of Mount Mercer, dated Sun 2nd Aug 31. .21) Handwritten three page letter regarding the Tunnel progress, the lease agreement on Mr Read's property at Mount Mercer which he had been unable to pay and a parsley root remedy which a Ballarat Chinese herbalist had prescribed for his catarrh and had been effective, dated Thur 13th Aug 31. .22) Handwritten two page letter regarding the efficacy of the parsley (root) water in healing his catarrh and detailed progress report on the Tunnel which showed a little gold in the uncovered 'wash', dated Mon 24th Aug. .23) Handwritten three page letter regarding the progress of the Tunnel and one of the bores sunk also mentions his health, dated Mon Sept 7th. .24) Handwritten three page letter regarding the Tunnel progress in detail and an account of his illness, dated Thur 24th Sept. .25) Handwritten two page letter regarding the Tunnel progress in detail and his health which had been poor, dated Thur Oct 8th 31. .26) Handwritten four page letter regarding the Tunnel progress in detail, dated Thur Oct 22. .27) Handwritten four page letter regarding a detailed report of the Tunnel progress including the news of some show of gold and other personal matters including advising Tom not to drive there when he would have to drive home in the dark because of the accidents caused by 'Boosy Drivers', dated Thur Nov 19th 31. .28) Handwritten two page letter regarding the continued promising Tunnel progress, dated Thur Nov 26th. .29) Handwritten two page letter regarding Tunnel progress and personal matters relating to the coming Christmas visit, dated Thur Dec 3rd 1931. .30) Handwritten two page letter regarding Tunnel progress and personal matters regarding the impending visit by the family, dated Sun Dec 6th 31. .31) Handwritten two page letter regarding his health and the treatment proscribed by a Chinese herbalist and also some information about his expenses, dated Sunday 25/1/32. .32) Handwritten four page letter regarding Tunnel progress including a small diagram and further information on his health, dated Thur 25th Feb 32. .33) Handwritten three page letter regarding Tunnel progress and some personal and family information, dated Mon 21st 32. .34) Handwritten one page letter regarding some personal matters and information about the Tunnel progress, dated Thus 31st Mar. .35) Handwritten two page letter regarding detailed information about the Tunnel as well as a one page diagram of the Drives being excavated, dated Sun night 3rd April. .36). Handwritten three page letter from his home in Prahran regarding a mixture of personal matters and matters relating to the Tunnel, dated 18/4/32. .37) Handwritten one page letter regarding enclosed three shares which were to be placed as he was very short of funds, dated 19-4-32. .38) Handwritten two page letter from his home in Prahran regarding work carried out by Ned at the Tunnel and family matters, dated 6/5/32. .39) Handwritten one page letter from his home in Prahran regarding work carried out by Ned at the Tunnel, dated 10/5/32 plus an attached one page letter written by Ned (E. Woodlook) to 'Mr Squire' regarding regarding Tunnel progress, dated Saturday. .40) Handwritten two page letter from his home in Prahran regarding Tunnel progress and some personal matters, dated 27/5/32 plus an attached one page letter written by Ned to 'Mr Squire' regarding Tunnel progress and with the information that the rats were bad in Richard's hut, dated Friday. .41) Handwritten two page letter from his home in Prahran regarding Tunnel progress, dated 3/6/32 plus a two page letter written by E.Woodlock to 'Mr Squire' regarding Tunnel progress as well as person matters regarding his health, dated Friday (27th May 32 written in pencil by Richard). .42) Handwritten three page letter from his home in Prahran regarding tunnel progress and financial matters, also detailed information about the Madison's Tunnel, dated 8/6/32, plus a one page letter from E. Woodlock (Ned) to 'Mr Squire' regarding Tunnel progress dated Saturday (4 June, 32). .43) Handwritten three page letter from his home Prahran regarding a sketch he had made of Madison's Tunnel and the Mercer Shaft (not present) and the similarity of other mines with barely Payable gold, dated 13/6/32. plus a one page letter from E. Woodlock to 'Mr Squire' regarding Tunnel progress dated Saturday. .44) Handwritten one page letter by Richard Squire to 'Tom' from his home in Prahran with some personal information as well as the hope to return to Mt Mercer as he felt he was now well, not dated, plus a two page letter from E. Woodlock to 'Mr Squire' regarding duty stamps sent and Tunnel progress dated Saturday. .45) Handwritten two page letter from his home in Prahran regarding the progress of the Tunnel, dated 21/6/32, plus a one page letter from E. Woodlock regarding the progress of the Tunnel work, dated Monday (20/6/32). .46) Handwritten three page letter from his home in Prahran regarding the price of gold and the effect that mining Payable gold in the Madison Tunnel could have, dated 27/6/32. .47) Handwritten three page letter by Richard Squire to Tom, Hazel & Kiddies all' from his home in Prahran regarding his thoughts on the Madison Tunnel at Piggoreet, dated Mon 4th July 32, plus two one page letters by E Woodlock to 'Mr Squire' regarding the Tunnel work and more personal things, dated Wednesday (June 29th 32) and Saturday (July 2nd). .48) Handwritten two page letter from his home in Prahran regarding his intention to return to the diggings in a small car procured for his use and his intention to re-peg the Leigh River Lease in another name, dated Sun 17/7/32, plus a two page and a one page letter by E Woodlock to 'Mr Squire' regarding progress at the Tunnel dated Thursday (7th July) and Tuesday (12th July 32). .49) Handwritten one page letter by E Woodlock to Mr Squire detailing the tunnel work and other work related details dated Saturday (16th July 32), plus a one page letter by 'Dad' (Richard Squire) to 'Tom, Hazel & Kiddies all' regarding the letter sent by Ned (E Woodlock) and the ongoing work. He also talks about the health of Mam, his wife, dated 19th July 32. .50) Handwritten one page letter regarding the work at the tunnel and with the information that he was to finally return to the mine, dated 22/7/32, plus a one page letter by E Woodlock to Mr Squire about the ongoing work, dated Wednesday (20th July). .51) Handwritten two page letter written from Mt Mercer, regarding the works in the Mt Mercer Tunnel (mentioning South Cockloft). He also details that the drive from town (Melbourne) took 4 gallons of petrol, dated Thur 28th July 32. .52) Handwritten 3 page letter detailing the work carried on at the tunnels and with the information that underground gas had halted work temporarily. He was keen that Tom should visit one weekend soon, dated Sat 6th Aug 1932. .53) Handwritten four page letter detailing the work and new bore holes near Madisons Tunnel. The No 1 tunnel work had to be suspended due to continued gas filling the diggings every time the barometric pressure dropped. On the last page was a rough sketch of the area which was being worked, dated 19th Aug 32. .54) Handwritten two page letter regarding Tom's proposed visit to the site and some plans that he should bring with him. He also spoke of a pup that he was housing till Tom came, dated 21/8/32. .55) Handwritten one page letter encouraging Tom to bring skid chains for his vehicle as the road was muddy when he came on the weekend. He gave a brief account of the work and of the pup's progress, dated 25/8/32 .56) Handwritten three page letter regarding the weather and the relief he felt at knowing Tom and his companions had arrived safely home. He also thanked Tom for the cheques for Ned and detailed a little of the work at the tunnel and the need for more explosives as well as the fact that they had had to put a lock on the door of the hut to stop intruders, dated Sun Sept 4th 32. .57) Handwritten one page letter regarding the ongoing work at the tunnel, dated Thur Sept 8/32. .58) Handwritten two page letter written from Prahran, regarding his trip home, work at the tunnel and with information about the enclosed receipts, dated 16/9/32. Also included was a one page letter from E Woodlock to Mr Squire regarding the work at the tunnel, dated Wednesday. .59) Handwritten one page letter from Dad (Richard Squire) to 'Tom, Hazel & Kiddies all' written from Mt Mercer, regarding the work at the tunnel and the effect that 2 inches of rain had on the work, dated Wed Sept 20th 32. .60) Handwritten two page letter regarding the work at the tunnel and with the information that Mr Read who owned the property where the tunnel was located, had a serious accident in Melbourne, dated Thur 29th Sept 30 (this 1930 date is a mistake as the information contained in this letter follows on from his previous letter dated 20th Sept 32). .61) Handwritten one page letter regarding the work at the tunnel with the encouraging information that flecks of gold were found in about half a dish (mining pan), dated Thur Sept 22nd. .62) Handwritten two page letter regarding the progress at the tunnel and the difficulties of the work and thanks for Ned's cheque. Also mentioned was the information that Mr Read had a fractured pelvis and would be in hospital for two months, so Mrs Read with her father was looking after the shearing, dated Thur 6th Oct. .63) Handwritten two page letter with a third page of a diagram of a cross section of the Leigh River Tunnel, including the new tunnel and Madisons tunnel, with detailed description of the work in the tunnel, dated Sat 8th Oct. .64) Handwritten two page letter written from Prahran, giving detailed information of the tunnel and the expected outcome of the work. He also commented that he was to visit Mr Read in hospital, dated 14/10/32. .65) Handwritten two page letter written from Mt Mercer, giving information about the work in the tunnel and his visit to Mr Read, dated Wed 19th Oct. Enclosed also was a one page letter from E Woodlock to Mr Squire giving an account of his work in the tunnel, dated Thursday. .66) Handwritten one page letter regarding the work in the tunnel, dated Sun Oct 23/32. .67) Handwritten two page letter from Dad & Jim (Richard Squire) to 'Tom, Hazel & Kiddies all' regarding the receipt of Ned's wages cheque and the insurance of Ned's person as well as a detailed description of the tunnel and its relationship to Madisons Tunnel. He also gives some indication of his health, dated Tue Nov 1st 32. .68) Handwritten on page letter written from Prahran, giving a small amount of information about the tunnel work as well as his visit to see Mr Read and some personal information, undated. Enclosed also is a one page letter by E Woodlock to Mr Squire about the work in the tunnel, dated Thursday. .69) Handwritten one page letter with little information, dated 11/11/32. Also enclosed is a one page letter from E Woodlock to Mr Squire about the work in the tunnel, dated Thursday. .70) Handwritten three page letter with detailed information about the tunnel work and an aside about Jim's help and the he suffered from "Imaginitis imagines he sees a speck of gold in every bit of gravel met", dated 10/11/32. .71) Handwritten two page letter with detailed information on the work as their tunnel crossed with the old Madison Tunnel, dated Thur 17th Nov. .72) Handwritten three page letter detailing information on an application for a 500 acre lease which was posted at the Grenville Post Office and how it would impact on their lease. The upshot was that their leases would need to be re-pegged and the fee to publish a Notice of Application on their Leigh River claim was necessary to pay and he wondered if one of their investors would pay the 10 pounds necessary to secure the claim. He also spoke of perhaps forming a Company to put a plant on their Mt Mercer shaft as he felt that the gold would be of a payable quantity, dated Sunday 20th Nov, Also included was a note written by Ned (Edward Woodlock) who had copied the Notice of Application. .73) Handwritten two page letter detailing information found in Madison's Tunnel as it related to their own tunnels, particularly No 2 tunnel and how far he felt he would need to tunnel to reach an improvement in the 'wash', dated Thur 24th Nov. .74) Handwritten one page letter thanking him for the cheque the investor, Mr Wilkinson had provided for the Notice of Application for the Mt Mercer old Lease of 828 acres. He also gave some information about how it was originally farm labourers who only worked this lease and only when they had no other work. He also gave some personal information about Mam's birthday, dated Mon Nov 28th 32. .75) Handwritten two page letter by Dad (Richard Squire) to 'Tom, Hazel & Kiddies 3' written from Prahran, telling them that they had received Ned Cheque and that that Jim and He had come home. He also detailed an incident that Jim had had with a tyre blowout whilst on his way to get explosives and post the Notice of Application in the Warden's Office in Ballarat. The stub axle had bent and Jim had to ride a bike to get a new one and after changing the inner tube of the tyre they were able to drive back to Melbourne and were in the process on having the tyre re-treaded, dated 6/12/32. .76) Handwritten one page letter explaining how he and Mam had contracted colds and that his kidneys had some of their of trouble. He hoped to return to Mt Mercer on the weekend, dated 18th Jan 33. Also included was a handwritten two page letter by E Woodlock to Mr Squire giving information about the tunnel work, an injury to his hand and the fact that he had only 3 picks that were any good. He also asked for some vegetables, tomatoes and bacon when Richard returned, dated Saturday. .77) Handwritten one page letter written from Mt Mercer, giving information about the tunnel work, now 97'6" in and how he had expected to have already come upon the Madison's gold bearing gravel wash, dated Wed 25th Jan 33. .78) Handwritten two page letter with detailed information about how the tunnel had cut across a second Madison's tunnel and the prospects in this tunnel looked more promising. He also stated that he had not been well the last few days, dated Frid 27th 33 .79) Handwritten one page letter with information about the shotty gold found and the tunnel work and that he would test the value of the wash where the gold was found, dated Mon 30th Jan. .80) Handwritten short note of one page giving sketchy information about the tunnel work, dated Tue 31st Jan 33. .81) Handwritten two page letter giving information about his dealings with the Secretary for Mines relating to the fact that because the application for lease was identical to the old lease, they therefore should not need to pay for a full survey costing 7 pounds, just an inspection. He also detailed the workings and asks for more parsley roots to be sent to him, dated Thur Feb 9th 33. .82) Handwritten one page letter stating that he had received Ned's cheque and information about the workings, the coarse gold found and the fact they were going home for fresh food, dated Tue 14th 33. .83) Handwritten two page letter giving detailed information on the progress of the tunnelling as well as the information that Len and Max had come to visit the site and that Len had brought with him Keating, who he detested and pondered the reason for his bringing the man. As they were leaving Max "told Ned he thought they were going down to Ice Mam". Richard was worried about paying the 7 pounds ten shillings for the Department of Mines survey and was loath to put in another 20 pounds for another share of the mine to pay for it. He gave his thanks for a parcel of parsley he'd received , dated Tue 21st Feb 33. .84) Handwritten two page letter written saying that he had received Ned's cheque (for wages) and giving detailed information on the progress and for the need to timber the drive and have the bottom stoped up. He had no timber or laths left and was concerned about the cost necessary to satisfy the Mines Department. He mentions the possibility of insolvency. Fine gold had been found but not like the Madison's tunnel, dated Tue 28th Feb 33. .85) Handwritten two page letter written giving detailed information of the progress in the tunnel and with the expectation that they would soon meet the same wash which was in Madison's Tunnel. He also personal information about his health. the parsley roots received and the apples which Jim had "burgled", dated Sun 5th Mar 33. .86) Handwritten four page letter by with a very detailed account of the progress of the tunnelling and the reason why the expected intersection with the Madison wash did not occur, but with the hope that this intersection would soon occur, then they would be able to meet expenses. He also talked about not being able to pay for the lease but had the hope they they would not be too rigid in their case. He added some personal information about Jim not having the makings of a miner, catching rabbits "for the pot" and the fact that Mr Read was so much better that he was able to ride his horse, dated Sat 11th Mar 33. .87) Handwritten one page letter written from Prahran, acknowledging the receipt of Ned's cheque, a note about the work at the tunnel and some personal information, dated 21/3/33. .88) Handwritten one page letter written from Mt Mercer, saying that there was little change in the tunnel but that he was would open a cross cut north, dated Thur 23 March 33. .89) Handwritten note of a half page telling them that he had cross cut the tunnel, dated Sat 25th Mar. .90) Handwritten one page letter with progress of the tunnelling and where it is in relation to Madison's Tunnel, dated Thur 30th Mar 33. .91) Handwritten three page letter with the first part of the letter talking about the personal and financial worries he and his family had with travelling and mining expenses as well as Ned's wages which could not have been managed without Judy's little car, Jim's help and the payment of Ned's wages by Tom. He went on to give detailed information of the tunnelling and then talked about a letter received by the Department of Mines regarding the non payment of the lease and that a Notice of Abandonment would be published if not paid. Lastly he talked about the struggle he had had with this process and that it was only because of Tom's help that he had been able to continue this far, dated Sun 2/4/33. .92) A one page letter with information about the progress as well as the information that he had not heard from the Mines Department regarding the lease, and the the 1000 sq ft Miners Right Claim was secure, dated Thur 6th April. .93) A three page letter with detailed information about the work in the tunnel and also detailed explanation of the leases he has pegged and repegged. He also thanked Tom for paying the balance which was owed to the Department and informed Tom that he was going home, dated Tues 11th April 33. .94) A four page letter written from Prahran, thanking Tom for Ned's cheque and with information about the work still being carried on by Ned. He also talked about the pegging of the Mt Mercer claim and the cost of the advertisement and application and survey fees to secure the site as well as his opinion of the probable value of the gold from this site. He also stated that he really needed more investment from those who had initially invested with him or from new investors, dated 17/4/33. Included was a one page letter from Edward Woodlock (Ned) to Mr Squire regarding the work he was carrying out at the tunnel, dated Saturday. .95) A three page letterwritten from Mt Mercer, regarding the work in the tunnel and the quality of the gold found and the direction they will take. He also stated that he was posting letters to the original investors to see if they would contribute to the cost of the lease and also talked about another man who had a Notice of Application posted at Grenville for the water rights for a 25 miles long area and a dam. His Capital is 300 pounds and the supposition is that he wants to 'unwater' the leads, dated Frid 20th April. .96) A one page letter regarding the continued work in the tunnel and how his suppositions seemed to be correct, dated Thur 27th April 33. .97) A three page letter thanking Tom for Ned's cheque of 6 pounds 7 shillings and 6 pence. He also gave detailed information about the tunneling and the type of ground found and his next intentions. He also gave further information about the Notice of Application at Grenville which was posted by B Ryan, Agent for Western Deep Leads Coy Limited for 6000 acres, dated Sun 30th April 33. As well is a note detailing the information copied from the Notice. .98) A two page handwritten letter detailing the work being carried out as well as a complaint that he had not heard from the men he had written to, dated Thur 4th May 33. .99) A one page handwritten letter regarding the tunnel work and informing Tom that the gold found is shotty, dated Sat 6th May 33. .100) A four page handwritten letter written from Prahran, detailing the tunnel work and informing that the gold prospects were better in no.1 cross cut south and there was also payable fair gold where they were currently working, if worked in bulk. He also talked about the possibility of new investors as the old ones had not responded to his letter and the necessity of securing the leases, dated 11th May 1933. .101) A one page handwritten letter by E. Woodlock (Ned) to Mr Squire regarding the work going on, dated Thursday. .102) A six page handwritten letter written from Prahran historically detailing the acquisition of the two leases at Mt Mercer, 35 years earlier, with M C Donnely/Donney and Jas Clements, including Madisons. He goes on to clarify Tom's suggestion regarding the Leases and the Companies to be floated, then details the the shafts, bores, tunnels and Deep Leads held in the leases and then goes on to say what his next steps would be, dated 13th May 33. .103) A two page letter written from Mt Mercer detailing the continued work in the tunnel with the added information that because of the incline it was taking two men to push the truck up the tunnel. Richard also told of the need to take more parsley water for his condition, dated Thur 18th 33. .104) A three page letter with a very detailed description of the work in the tunnel. Richard also told that he had run out of metal rails and was having to use timber as a substitute. He was also to re-peg the North and South Leases in the morning, dated Sun 21st May 33. .105) A two page letter detailing the work in the tunnel and saying that there was a hundred feet of rail locked in by a fall in the No.2 Tunnel and he was hoping to get them out to replace the wooden rails as they made for heavy work on the inclines. He also said that he had a letter from the Department telling him to communicate with the Surveyor in Ballarat, dated Wed 24th May. .106) A three page letter acknowledging Ned's cheque as well as information about the work in the tunnel, including that he had been able to retrieve 45 feet of rail and would get more when needed. He said he was pleased that Tom and Mr Wilkinson were making a trip on Saturday to see him. He talked of the weather and the fact that the bread was a week old, so to bring enough to last till the Monday. Among other things he also spoke of Tom revising the Plans and also having a Share book printed and the name was to be the Ballarat Deep Leads Extension for which there could possibly be 3 Companies, dated Sun 28th May. .107) A one page letter with information about the work in the tunnel and also a weather update and the best way to come, dated Wed 31 May 33. .108) A two page letter written from Prahran, explaining a visit to the Leigh River Shaft to get whim rope, whip wheels and sundry items. He shifted some equipment and built a new forge and was to fix and mount a windlass and rope to enable Ned to get the truck up the incline of the tunnel. He also spoke of the work being undertake by Ned then when on to more personal correspondence about a birthday present for one of Tom's boys, dated 9/6/33. .109) A two page letter acknowledging receipt of the 'Prospectus of the Ballarat Deep Leads Extension Syndicate' and 'Share Certificate'. The No.1 Lease was in Ned's name and the No.2 Lease was in Jim's name to avoid inquisitive interest. Jim logged a Notice of Application and paid the fees. Richard acknowledged receipt of Ned's cheque and asked for 5 pounds as his finances were 'rather tight', dated 14/6/33. Included was a 1 page letter from Ned to Mr Squire regarding the work in the mine, dated Saturday. .110) A one page letter written from Mt Mercer giving information about what was happening in the new x cut N near the mouth of the tunnel, dated Friday 16/6/33. .111) A one page letter telling about the work and that he had fixed an old shed of Mr Reads for the forge. Mr Read and Mr Cameron visited and Richard was hopeful that Mr Cameron, the owner of the land in the North Lease would be easy as regards an agreement, dated June 22/33. .112) A two page letter which talked about his health, the mine, the local J.P. who had lost his eye in a shearing accident and the surveyors visit, He also spoke about contacting the Gold Mines Ltd and the Berry Leads Company, dated Tue 27th 33. Also included was a letter to the Mines Department and a receipt from the Mines Department for the sum of 7 pounds 10 shillings as well as the Lease Applications from the newspaper dated June 13th 1933. .113) A two page letter complaining of the charge made by the Mines Department for a Surveyors Inspection. He also wrote of trying to set up a float for the mine which he would do when he returned home.He also explained the current findings at the mine, dated Sun 2nd July 1933. .114) A one page note written from Prahran informing Tom that he had not yet heard from Gold Mines Ltd and also that there was a hitch with the lease on the house, but this was to hopefully be fixed the following day. He also spoke of Tom's trip home (completed in tow), dated 18/7/33. .115) A two page letter recounting some of the difficulties they were having with the new owners of the house they leased in Prahran. He also spoke of the fact that the Mines Department had requested another 10 pounds although they had not yet completed the survey on either mine, dated 21/7/1933. Also included was a one page letter from E. Woodlock (Ned) to Mr Squire about the workings, dated Tuesday and a letter from the Gold Mines of Australia Limited saying that the information he had supplied was now with their engineers, dated 20th July, 1933. .116) A three page letter written from Armidale saying that Mam (his wife) was fretting with the move to this house. He also told of the rejection by the Gold Mines of Australia Limited of his proposal to invest in his Mt Merser Mine, stating that he believed that Jim Clements who had been the manager when the mine had previously been opened, had most likely "thrown all the cold water he could" on the proposal. He spoke at length about how this man had run the mine into the ground and his own involvement with the winding up of the company. He went on to say that he would approach Ryall to see if he was interested in investing, dated 28/7/33. Included was the letter from Gold Mines of Australia Limited, dated 26th July, 1933. .117) A two page letter written from Prahran telling of the move to another house where Mam was quite settled. He also wrote of the work Ned was carrying out at the mine as well as personal and family news. He wrote that as he had not heard from Ryall, he would visit him the following day, dated 6/8/33. Also included was a two page letter from E Woodlock to Mr Squire giving detailed information of the progress at the tunnel, as well as asking for a new pair of boots as the ground was so wet his were letting in the water, dated Thursday. .118) A two page letter explaining about a letter he received from Mr Cameron who believed he had found gold as well as some personal information and his health, dated 8/8/33. Also included was a one page letter from Neil W Cameron to R B Squire telling about some specks of gold he'd found in a post hole, dated July 31st 33. Thirdly was a one page letter from Ned to Mr Squire explaining the work he was doing in the tunnel, dated Saturday. .119) A one page note saying that he did not like the enclosed 2 copies of the typed Agreement of the Leigh River Gold Mining Company with William Ryall, but asked Tom's advice. dated 9/8/33. .120) A two page letter dealing mainly about the work going on at the new house and the settling in process. He also wrote about the proposed Agreement with Ryall, dated 11/8/33. Also included was a one page letter from Mr Ryall to R B Squire from which part of the left hand side had been severed, dated 10th August 1933. .121) A one page note from R B Squire to W Ryall dated 14/8/33, with attached one page of information and figures. Also attached a one page letter from R B Squire to W Ryall asking if Ryall would be interested viewing the plans for an alluvial mining proposition, dated 27/7/33. .122) A three page letter mainly about the visit to Ryall and a Mr Cundy about the proposed agreement with his concerns about the language and terms of the agreement, dated Sun 21st 33. Also included was a letter from Ned to Mr Squire about the continued work in the tunnel and the fact that two of the pick heads had cracked and would have to be fixed, dated Thursday. .123) A two page letter dealing with a meeting had with Ryall and others in a private office regarding the interest in investing in the mining proposition, dated 23/8/33. .124) A four page letter dealing mainly with the work at the tunnel and exploratory diggings at the spot Mr Cameron had written about as well as the damage done to the car on the way to the workings, dated 29/8/33. .125) A one page letter telling of a proposed meeting with Mr Taylor and a Mr Bowler, who may turn out to be Alan Bowler who he knew, regarding the Mr Mercer Float scheduled for the following day, dated 30/8/33. .126) A five page letter explaining the content of the meeting held with Mr Bowler, who represented an English consortium which was interested in the Mt Mercer site. He explained the terms that the consortium would offer if they went ahead, This included 25 % shares in the mine but no money, which he bemoaned because of the shortness of their cash reserves. He went on to detail the 4 distinct deposits of wash in this field, which he believed were unique in the Ballarat district. He also stated that he had not heard from Mr Ryall. He closed by talking about the water levels in the mine, dated 1/9/33. .127) A two page letter saying that he had written an 11 page report on the Mt Mercer field for the English consortium as well as a private letter for the chairman.He also reported that he had been to see Ryall. Neither party knew that he was talking to the other. added was some personal information, dated 6/9/33. .128) A one page letter telling of a meeting with Mr Bowler and Mr Tayler who carefully went through his report. It was then being typed. He believed he had two strong supporters, dated 7/9/33. Also included was a 2 page note from A Bowler to R B Squire asking him to bring the report into the office the following day to be perused and typed, dated Sept 6th 33. .129) A two page letter with personal encouragement for one of the children. The letter then goes on with more information about the meeting with Mr Tayler and Bowler and his impressions of their interest. He also talked about the one of the experts who was to examine the site and also about the report which was now typed and a copy sent to Tom, dated 8/9/33. .130) A one page letter keeping him informed of progress. The expert was away but would be briefed on his return. He also thanked Tom for a cheque, which was used to get the car 'Liz' back in order for the trip to be made with the experts at a later date. He informed Tom of his intention to return to Mt Mercer, dated 13/9/33. .131) A one page letter written from Mt Mercer telling of the trip from Melbourne and the weather. He spoke of what was happening in the tunnel and that he had not yet heard from any of the interested parties, dated Tue 19th Sept. .132) A five page letter written from Prahran with detailed information about the tunnels, the washes and the shows. He also detailed his meeting with Bryant 38 years ago at the No 1 at Carisbrook and how he had given him assistance at that time and subsequently as well as giving his version of a potted history of Bryant, dated 29/9/33. .133) A two page letter with information about the arrival of the chairman of the English group and that Ned had shown Mr Kermode around the site, dated 6/10/33. Also included was a cutting from the Age about the arrival of Mr F W Baker, representing a large English financial group interested in Victorian deep lead propositions, dated Oct 6th. As well were two, one page letters from Ned (E Woodlock) to Tom, regarding the work in the tunnels, the bad air and the hopes of a successful float, dated Tuesday and Wednesday. .134) A one page letter thanking them for the gift of eggs and parsley. He also talked about a letter from Bowler regarding the business of the experts and that their leases would be seen in due course, dated 10/10/33. Included was a one page letter from A Bowler to P B Squires saying that Mt Mercer site was receiving attention but that no decision had as yet been made, dated Oct 9th 1933. .135) A three page letter written from Prahran giving information about Richards visit and conversation with Mr Ryall, who indicated that no decision had as yet been made regarding his proposal. He also included some personal information about members of the family, dated 18/10/33. Included was a one page letter from Ned (E Woodlock) to Tom with information about the tunnelling progress in no.2 tunnel and the bad air that drove them out for a time, dated Saturday. .136) A one page letter containing a brief update on the state of affairs , dated 20/10/33. Included was a one page letter from Ned to Mr Squire with an information on the progress at the tunnel and with the information that his hands were cracked so badly that he was wearing two socks on each hand, dated Saturday. .137) A one page letter which family news and a paragraph about the figures in his calculations, dated 21/10/33. On the back side was a letter from E Squire (his wife) to Tom, Hazel & boys again with family and personal information, dated 22nd 10/33. .138) A three page letter with the disappointing news that the English consortium would not take up the option on the Mt Mercer lease and gave his opinion that it was self interest that stopped it going ahead. He then gave two options as to the way forward, dated 24/10/33. Also included was a typed one page letter from W C Tayler to R B Squire informing him of a letter stating that the proposal had been turned down, dated 23rd October 1933. .139) A three page letter informing of further developments with Mr Tayler who asked for the plans to be left at the office till the following week as there was another company who may yet be interested to take up the option, dated 27/10/33. .140) A one page letter asking for 9 copies of the old agreement to be typed for the 3 Syndicate members, Rice, Cameron and McNaughton, dated 5/11/33. .141) A three page letter regarding the decision by the Syndicate to reopen the LRG shaft and the practical issues of timber acquisition and probable costs. He then went on to talk about Mam's indisposition and treatment by the herbalist, Goon, dated 8/11/33. Also included was a newspaper clipping from the Age entitled 'Inquiries for properties at Ballarat'. .142) A three page letter giving all the news regarding the agreements, the costs and the way forward. He also spoke of his wife's improvement and other personal information, dated 11/11/33. .143) A two page letter written from Mt Mercer regarding the difficulties in obtaining the timbers and tanks needed to reopen the shaft and the state of all the existing fittings and what would be needed to get the site operational again. His agreement with Mr Read had duly been signed, dated Thurs 16th 33. .144) A two page letter with further information about the progress with acquiring and repairing the shaft site, dated Mon Nov 20th 33. .145) A three page letter informing that the timber for the whim & shaft were being delivered and the area had been cleared out for the poppet heads. The top of the shaft had been cleared ready for re-framing and other work had been carried out. Liz, the car had broken down and Jim was in the process of fixing her. He concluded with information about his and his wife's health, dated Sun 26th Nov 33. .146) A four page letter with news of Mam's health, the problems associated with fixing the car and the floods which stopped Jim from going to Ballarat and washed away the Gary Bridge which affected the mail and had halted work at the shaft, as well as delaying the second delivery of timber, dated Sun Dec 3rd. .147) richard squire, william ryall, jim clements, gold mines of australia limited, e woodlock, ned woodlock, neil w cameron, alan bowler, mr cundy, don mcnaughton, mr rice -
 Flagstaff Hill Maritime Museum and Village
Flagstaff Hill Maritime Museum and VillageDomestic object - Plate
The Process of Making Pottery Decorating, Firing, Glazing, Making, Technical There is a rhythm and flow to clay. It can’t be done all at once! Even the making process! It can take weeks to get everything done, especially if you can only work on your pottery once a week! Even though we have three hour classes, it’s often just not enough time! Here is an overview of some of the processes so you have a bit more grasp on some of the technical stuff! Step One – Design There are SO many ideas out there for making stuff in clay! From delicate porcelain jewellery, through to heavy sculptural work and everything in between. Deciding your direction is sometimes not that easy – when you first start, try everything, you will naturally gravitate to the style that you enjoy! The options and variations are endless and can get a wee bit overwhelming too! Check in with me before you start to ensure your ideas will work, what order you might do things, how you could achieve the look you are seeking and any other technical data required! Step Two – Making Clay is thixotropic. This means that as you work with it, the clay first gets sloppier and wetter, before is begins to dry in the atmosphere. For most things, you simply can’t do all parts of the project at once. An example of work order might look like: Get last weeks work out from the shelves Prepare clay for today’s work – roll your clay, prepare balls for throwing, make the first stage of a pinch pot) Clean up last week’s work and put it on the shelf for bisque firing Check that you have any glazing to do – and do enough of it that you will have time to finish your main project Do the next step of your next project – there might be a further step that can’t be complete immediately, in that case, wrap your work well and put onto the shelves. Letting your work rest for a while can really help keep your work clean and professional looking. Many things require bagging under plastic to keep it ready for work the next week – put your name on the outside of the bag so you can find your work easily. We have stickers and markers. Consider how you want to decorate your work – coloured slip can be applied at a fairly wet stage (remembering that it will make your work even wetter!). Trying to apply slip to dry clay won’t work! If you want to do sgraffito – you will need to keep the work leather hard (a state of dryness where you can still work the clay with a little effort and a little water and care). Step Three – Drying Most of the time your work can go into the rack uncovered to let it dry out for the following week. If you want to continue forming or shaping you will need to double bag your work – put your work on a suitable sized bat and put the bat in a bag so the base of the bag is under the bat, then put another bag over the top of the work and tuck the top of the bag under the bat. If you want to trim (or turn) your thrown work the following week, it should also be double bagged. If your work is large, delicate, or of uneven thicknesses, you should lightly cover your work for drying. When considering the drying process, bare in mind the weather, humidity and wind! The hotter and dryer, the faster things dry and work can dry unevenly in the shelves – this can lead to cracking – another time to lightly cover your work for drying. Step Four – Trimming and Cleaning Up Your work is dry! It is called greenware now and it is at it’s most fragile! Handle everything with two hands. I often refer to soft hands – keep everything gentle and with your fingers spread as much as possible. Try to not pick up things like plates too much, and always with both hands! Before your work can be bisque fired it should be “cleaned up”. You work won’t go into the kiln if it has sharp edges – when glazed, sharp edges turn into razor blades! Use a piece of fly wire to rub the work all over – this will scratch a little so be light handed. Use a knife or metal kidney to scrape any areas that require a bit more dynamic treatment than the fly wire offers! Finally, a very light wipe over with a slightly damp sponge can help soften and soothe all of your edges and dags! Trimming thrown work: If you are planning to trim (or turn) your thrown work (and you should be), make sure you bag it well – your work should be leather hard to almost dry for easiest trimming. Use this step to finish the work completely – use a metal kidney to polish the surface, or a slightly damp sponge to give a freshly thrown look. Wipe the sponge around the rim after trimming, and check the inside of the pot for dags! Trimming slip cast work: Usually I will trim the rims of your work on the wheel the following day to make that stage easier, however you will still need to check your work for lumps and bumps. Last but not least – check that your name is still clearly on the bottom of your work. Step Five – Bisque Firing When the work is completely dry it can go into the bisque kiln. The bisque kiln is fired to 1000°C. This process burns off the water in the clay as well as some of the chemically bound water. The structure of the clay is not altered that much at this temperature. Inside the bisque kiln, the work is stacked a little, small bowl inside a larger bowl and onto a heavy plate. Smaller items like decorations or drink coasters might get stacked several high. Consideration is paid to the weight of the stack and shape of the work. A bisque kiln can fire about one and a half times the amount of work that the glaze kiln can fire. The firing takes about 10 hours to complete the cycle and about two days to cool down. Once it has been emptied the work is placed in the glaze room ready for you to decorate! Step Six – Glazing Decorating your work with colour can be a lot of fun – and time consuming! There are three main options for surface treatment at this stage: Oxide Washes Underglazes Glazes Washes and underglazes do not “glaze” the work – It will still need a layer of glaze to fully seal the clay (washes don’t need glaze on surfaces not designed for food or liquid as they can gloss up a little on their own). Underglazes are stable colourants that turn out pretty much how they look in the jar. They can be mixed with each other to form other colours and can be used like water colours to paint onto your work. Mostly they should have a clear glaze on top to seal them. Oxides are a different species – the pink oxide (cobalt) wash turns out bright blue for instance. They don’t always need a glaze on top, and some glazes can change the colour of the wash! The glazes need no other “glaze” on top! Be careful of unknown glaze interactions – you can put any combination of glaze in a bowl or on a plate, but only a single glaze on the outside of any vertical surface! Glazes are a chemical reaction under heat. We don’t know the exact chemicals in the Mayco glazes we use. I can guess by the way they interact with each other, however, on the whole, you need to test every idea you have, and not run the test on a vertical surface! Simply put, glaze is a layer of glass like substance that bonds with the clay underneath. Clay is made of silica, alumina and water. Glaze is made of mostly silica. Silica has a melting point of 1700°C and we fire to 1240°C. The silica requires a “flux” to help it melt at the lower temperature. Fluxes can be all sorts of chemicals – a common one is calcium – calcium has a melting point of 2500°C, however, together they both melt at a much lower temperature! Colourants are metal oxides like cobalt (blue), chrome (green through black), copper (green, blue, even red!), manganese (black, purple and pink) iron (red brown), etc. Different chemicals in the glaze can have dramatic effects. for example, barium carbonate (which we don’t use) turns manganese bright pink! Other elements can turn manganese dioxide brown, blue, purple and reddish brown. Manganese dioxide is a flux in and of itself as well. So, glazes that get their black and purple colours, often interact with other glazes and RUN! Our mirror black is a good example – it mixes really well with many glazes because it fluxes them – causes them to melt faster. It will also bring out many beautiful colours in the glazes because it’s black colouring most definitely comes from manganese dioxide! Glaze chemistry is a whole subject on it’s own! We use commercial Mayco glazes on purpose – for their huge range of colour possibilities, stability, cool interactions, artistic freedom with the ability to easily brush the glazes on and ease of use. We currently have almost 50 glazes on hand! A major project is to test the interactions of all glazes with each other. That is 2,500 test tiles!!!! I’m going to make the wall behind the wheels the feature wall of pretty colours! Step Seven – Glaze (Gloss or sometimes called “Glost”) Firing Most of the time this is the final stage of making your creation (but not always!) The glaze kiln goes to 1240°C. This is called cone 6, or midrange. It is the low end of stoneware temperatures. Stoneware clays and glazes are typically fired at cone 8 – 10, that is 1260 – 1290°C. The energy requirement to go from 1240°C to 1280°C is almost a 30% more! Our clay is formulated to vitrify (mature, turn “glass-like”) at 1240°, as are our glazes. A glaze kiln take around 12 hours to reach temperature and two to three days to cool down. Sometimes a third firing process is required – this is for decoration that is added to work after the glaze firing. For example – adding precious metals and lustres. this firing temperature is usually around 600 – 800°C depending upon the techniques being used. There are many students interested in gold and silver trims – we will be doing this third type of firing soon! After firing your work will be in the student finished work shelves. Remember to pay for it before you head out the door! There is a small extra charge for using porcelain clay (it’s more than twice the price of regular clay), and for any third firing process! Once your work has been fired it can not turn back into clay for millennia – so don’t fire it if you don’t like it! Put it in the bucket for recycling. https://firebirdstudios.com.au/the-process-of-making-pottery/Ceramics have evolved over thousands of years.White earthenware dinner plate. Crazing evident all over.Backstamped ‘Made in England S LTD’flagstaff hill, warrnambool, shipwrecked-coast, flagstaff-hill, flagstaff-hill-maritime-museum, maritime-museum, shipwreck-coast, flagstaff-hill-maritime-village, ceramics, tableware
