Showing 88 items matching "calcium"
-
 City of Moorabbin Historical Society (Operating the Box Cottage Museum)
City of Moorabbin Historical Society (Operating the Box Cottage Museum)Containers, cardboard box 'D.C.P. WAFERS', mid 20thC
‘D.C.P WAFERS’ made by Parke, Davis & Co Pty Ltd Sydney, that contained Calcium and Phosphorous. The wafers ‘could be chewed or allowed to dissolve in the mouth’ and ‘ were agreeably flavoured with Chocolate’An empty cardboard box, with a sliding inset, for ‘D.C.P WAFERS’ that contained Calcium and Phosphorous. The wafers ‘could be chewed or allowed to dissolve in the mouth’ and ‘ were agreeably flavoured with Chocolate’Top: 36 WAFERS / MEDICAMENTE VERA encircling PDCo. / D.C.P. WAFERS / (DICALCIUM PHOSPHATE) / AGREEABLY FLAVOURED / WITH CHOCOLATE / EACH WAFER CONTAINS DICALCIUM PHOSPHATE 15 GRAINS / FOR DIRECTIONS SEE REVERSE SIDE / PARKE, DAVIS & CO. / SYDNEY . Left and Right Sides: D.C.P. WAFERS. Base: D.C.P. WAFERS …… same ratio as … milk … soluble … Indicated during periods of growth …../ DIRECTIONS / ….. They may be chewed or allowed to disintegrate in the mouth….pharmacy, parke-davis pty.ltd, moorabbin, cheltenham, bentleigh, disease, bone disease, osteoporosis, calcium -
 Musculoskeletal Health Australia (now held by the Glen Eira Historical Society)
Musculoskeletal Health Australia (now held by the Glen Eira Historical Society)Photograph - Group photo, 2001
In 2001, a new national Osteoporosis Australia campaign, "Healthy Bones, Healthy Body", was launched. In this photo, Australian long-distance runner, Steve Moneghetti, and two children pose with our skeleton mascot, Bridget Bones, to promote the campaign. The photo appeared on page 9 of the Vol 14 Issue 03, Spring 2001 issue of the Arthritis Foundation of Victoria's quarterly magazine, Update. It accompanies a brief article titled, "moneghetti promotes calcium for kids".COL photo of a man and two children standing next to a plastic skeleton on a stand. They are each holding one fist up and smiling. The man is also holding up a packet of Kraft Singles cheese slices in his other hand. They are standing outdoors, with some leafy bushes in the background.[On a white label, handwritten in black ink] Steve Moneghetti promotes calcium for kids OA National campaign "Healthy Bones, Healthy Body" Update Spring 2001 [On a blue sticky label, handwritten in blue ink] Arthritis Update 2001 Vol. 14 Iss. 3 pg 9. Steve Moneghettiarthritis foundation of victoria, afv, osteoporosis australia, healthy bones healthy body, steve moneghetti, children, kraft cheese, kraft singles, calcium, update, 2001 -
 Musculoskeletal Health Australia (now held by the Glen Eira Historical Society)
Musculoskeletal Health Australia (now held by the Glen Eira Historical Society)Photograph - Group photo, May 2003
In May 2003, Arthritis Victoria (AV) and Osteoporosis Victoria (OV) had a stand at the Women's Expo. The stand had fact sheets and nutritional information available for women and girls to take home, as well as examples of fresh and packaged food items suitable for a nutritional and calcium-rich diet. This photo is a close up of AV staff and visitors at the AV/OV stand.Colour close-up photo of three women (two partially obscured) at an information stand. Headings on some printed factsheets on a display stand include "Bone Density and Osteoporosis", "Osteoporos and Excercise", and "Medications and..." (partially obscured). at an information stall. Two posters on the wall contain the text, "OSTEOPOROSIS VICTORIA Are you at risk of Osteoporosis? FIND OUT HERE". Between the posters, a small sign with the "OV" logo (for Osteoporosis Victoria) says, "Are you getting enough calcium? Surveys show most people don't!"arthritis victoria, av, osteoporosis victoria, ov, arthritis foundation of victoria, afv, womens expo, information stand, information booth, information stall, factsheets, bone health, strong bones, healthy bones, calcium, 2003 -
 The Beechworth Burke Museum
The Beechworth Burke MuseumGeological specimen - Fluorite (purple)
Fluorite comes in a variety of natural colours and crystal formations and glows under ultraviolet light (the word 'fluorescence' comes from the same etymological source). In its pure form, calcium fluoride, it is a colourless combination of the elements calcium and fluorine, but gains its colour from trace elements that infiltrate or replace calcium within its crystal structure during its formation. Although fluorite crystals polish well and can achieve a high level of lustre, the mineral is very soft (4 on MOHS hardness scale) so it is unsuitable for use in rings and must be handled and stored carefully if used in other forms of jewellery. Most crystals of the mineral are too coarse for decorative purposes but have been mined under the name fluorspar for a variety of commercial and industrial purposes. These include the production of hydrofluoric acid, smelting metal alloys, producing glazes and ceramic finishes and use in medical and dental products. An existing label for this specimen indicates that its origin or collection-point was 'probably USA.' Fluorspar, the form of fluorite used commercially and industrially, was mined in significant quantities in the counties of Hardin and Pope in South-Eastern Illinois throughout the Nineteenth and Twentieth Centuries. Fluorite was made Illinois' state mineral in recognition of its contribution to the state's identity and economy. The specimens are significant as examples of surveying activity undertaken to assess and direct the development of the mineral resource industries, as well as the movement to expand human knowledge of earth sciences such as mineralogy and geology in the nineteenth century.The specimen is a piece of purple shaded fluorite (also known as fluorspar), the mineral form of calcium fluoride. The unpolished specimen presents a dark purple interior with a substantial dark grey crust representing the matrix from which the specimen was obtained. Existing label: Flourite / (purple) / probably / USA / BB /burke museum, beechworth, geological, geological specimen, fluorite, flourite, calcium fluoride, hydrofluoric acid, jewellery, indigo shire, north-east victoria, mining, illinois, usa, united states, fluorine, gemstones, purple stones -
 Bass Coast Shire Council - Art Collection
Bass Coast Shire Council - Art CollectionArtwork, other - In my Father's House here are many Mansions, Michelle Watson
AustraliaWax medium, calcium carbonate oil on canvasSigned -
 The Beechworth Burke Museum
The Beechworth Burke MuseumGeological specimen - Dolomite
Dolomite is a mineral, calcium magnesium carbonate, with the chemical formula CaMg(CO3)2. It is a principle component of various rock types sometimes also referred to as dolomite, including dolostone, dolomitic marble and dolomitic limestone (according to the composition of each type). Dolomite rock is found in sedimentary basins throughout the world, comprising approximately 2% of the Earth's crust. It is formed when lime mud or limestone encounters groundwater containing magnesium. Dolomite can contain elements such as lead, zinc and copper. Dolomite and limestone are used in various construction, landscaping and agricultural processes. This specimen was donated to the Burke Museum in 1868 by Alfred Selwyn as part of the Geological Survey of Victoria. It was donated to the Museum in 1868. Victoria and other regions of Australia were surveyed for sites of potential mineral wealth throughout the 19th Century. The identification of sites containing valuable commodities such as gold, iron ore and gemstones in a locality had the potential to shape the development and history of communities and industries in the area. The discovery of gold in Victoria, for instance, had a significant influence on the development of the area now known as 'the goldfields', including Beechworth; the city of Melbourne and Victoria as a whole. Dolomite and limestone are mined at several locations in Victoria, including sites in the North-East of the state in Bindi and Limestone Creek. There are notable dolomite deposits in most Australian states. The dolomitised form of the mineral tends to come from older limestone deposits, formed during the palaeozoic era in marine settings, so this specimen may have come from a deposit located along a coastline in Victoria or another state. The specimen is significant as an example of surveying activity undertaken to assess and direct the development of the mineral resource industries in Victoria and Australia, as well as the movement to expand human knowledge of earth sciences such as mineralogy and geology in the nineteenth century. This specimen is part of a larger collection of geological and mineral specimens collected from around Australia (and some parts of the world) and donated to the Burke Museum between 1868-1880. A large percentage of these specimens were collected in Victoria as part of the Geological Survey of Victoria that begun in 1852 (in response to the Gold Rush) to study and map the geology of Victoria. Collecting geological specimens was an important part of mapping and understanding the scientific makeup of the earth. Many of these specimens were sent to research and collecting organisations across Australia, including the Burke Museum, to educate and encourage further study.Hand-sized piece of pale pink dolomite (calcium magnesium carbonate) with dark grey rim and hollowed centre. geological specimen, geology, geology collection, burke museum, beechworth, dolomite, mineralogy, geological survey, alfred selwyn, limestone, calcium magnesium carbonate -
 Coal Creek Community Park & Museum
Coal Creek Community Park & MuseumBox, cardboard, c.1937-1960
Trove : Advertised from 1937-1949 in various publications search under "Wellcome"' Calcium Borogluconate (yes with 2 'l's) . Victorian Government Gazette , no.2 Jan 5, 1960, page 16. List of Registered Stock Medicine. Registered Wholesale Dealer : Burroughs Wellcome and Co. (Aust) Ltd. Cressy Street, Rosebery New South Wales. Manufacturer, if other than the Wholesale Dealer - , Distinguishing Name of Stock Medicine : "Wellcome" Calcium Borogluconate, Approved Use or for the Treatment of : Milk Fever, hypocalcaemia. Rectangular faded pink cardboard box opening at both ends with the remnants of a paper label on one side, containing a folded paper leaflet and a cellophane bag containing white granules.Outer label '.....ATE .s enclosed)..........ELLCOME & .............STRALIA..D., SYDNEY, N....in Australia'. Impressed on one flap of box '132'. Printed leaflet (side one) Illustration of a unicorn, a thick black line under which text 'WELLCOME' brand CALCIUM BOROGLUCONATE (Vetinary)' followed by another thick black line. 'Calcium Borogluconate ia a stable , non-irritant calcium preparation for subcutaneous or intravenous injection in the treatment of milk fever and other forms of acute hypocalcaemia. It is available in the dry state as 'Wellcome' Calcium Borogluconate, a granular product in single dose containers of 2 1/2 oz. Milk Fever In the treatment of milk fever in cows, 21/2 oz. to 31/2 oz. of the granules should be injected subcutaneously at two or three points in the neck, with the usual aseptic precautions. The granules should be dissolved in 10 fl. oz. of boiling water, the solution allowed to boil for five minutes, then cooled to body temperature before administration. Repetition of the dose is very rarely necessary. Should a more rapid response be desired, the whole of the solution hay be given by slow intravenous injection; alternatively , the greater part of the solution may be injected by this route and the remainder given subcutaneously in the manner described above. A convenient apparatus for the controlled administration of large volumes of fluid (leaflet side two) is the 'Wellcome' Flutter Valve Injection Apparatus. Prophylaxis Recurrent attacks at successive parturitions may be prevented by giving calcium borogluconate immediately after calving and again about 20 hours later. Each dose should be from one or two ounces of 'Wellcome' Calcium Borogluconate, dissolved as directed above. Other Indications Certain other conditions have been found to respond readily to calcium borogluconate therapy. These include parturient hypocalcaemia or milk fever in ewes, parturient eclampsia in sows and bitches, so-called "staggers" in lactating dairy cattle suspected to be due to hypocalcaemia, and transit tetany in horses. The dosage for various species is generally within the ranges indicated below : horses and cattle 11/2 to 31/2 oz. Sheep, goats and pigs 1/2 oz. to 1 oz. Dogs 11/2 dr. to 3 dr. 'WELLCOME' brand CALCIUM BOROGLUCONATE A readily-soluble granular product issued in cartons of 21/2 oz.' Illustration of a unicorn, 'BURROUGHS WELLCOME & CO. (AUSTRALIA) LTD. (Incorporated in England) SYDNEY, N.S.W.' A black line 'ref.A5007g 54.1. 25' milk fever, hypocalcaemia, subcutaneous -
 The Beechworth Burke Museum
The Beechworth Burke MuseumGeological specimen - Calcite crystals
Calcite is a common mineral and is found worldwide due to it being a primary component of many other rocks such as limestone and marble. It is a softer mineral that scratches easily and is often found colourless or with a cream/white shade but may show up in colours such as red, yellow, green, and violet. In sedimentary rocks calcite is often found in the form of invertebrate shells, making it an important biomineral. Calcite is used in many industries such as farming, building, and medicine. This particular specimen was found at Broken Hill mine in Broken Hill, New South Wales, Australia. Broken Hill mine is one of the largest mines working silver and lead in Australia and at its peak employed 6500 staff across 7.5km long of land. The site was founded in 1883 by Charles Rasp, where Rasp and 6 other men from various backgrounds came together to form the first BHP mine. It has become one of the most popular mining sites due to its abundance and longevity. The ore body was created 1685 million years ago due to volcanic activity causing heated seawater to flow up through the seafloor where it mixed with the cold water creating black sulphide precipitates. These then settled back onto the seafloor forming sediment layers rich in minerals. Over time the land eroded until it was discoverable by humans.Historically this specimen is significant due to the origin of its location. Broken Hill mine has a long history in both its location and its findings and has resulted in a variety of minerals being discovered at its site. It is beneficial in the understanding of the Australian landscape over millions of years. Due to its properties, calcite today is used in a multitude of different industries such as agriculture, construction, medicine, and farming.A small sized calcium, carbon and oxygen made mineral specimen in shades cream and greycalcite, mineral, limestone, marble, sedimentary, invertebrate shells, biomineral, farming, medicine, broken hill, broken hill mine, new south wales, charles rasp, syndicate of seven, volcanic activity, black sulphide precipitates, calcite crystals, beechworth museum, indigo shire, beechworth -
 Federation University Art Collection
Federation University Art CollectionCeramic - Artwork - Ceramics, Stoneware Bowl by Robin Welch, 1980
Robin WELCH ( 23 July 1936-5 December 2019) Born Nuneaton, Warwickshire, England Robin Welch is one of the most highly respected contemporary British potters. The full range of his work includes large vessels with related paintings, fine drawings, and distinctive bowls and vases which explore colour, surface texture, form, detail of edge, and line. He is one of small group of significant British potters who expanded the language of throwing pots on the wheel through post-wheel additions and alteration. This gave his generally cylindrical forms a more organic and sculptural aspect, but their heavily coloured and textured surfaces were as much about painting, too, as Robin sought an integration of the visual disciplines he enjoyed. As he once wrote: “There’s no divide between art or craft. You decide to be an artist and you’ll use anything. If marooned on a desert island you’d use driftwood.” (https://www.theguardian.com/artanddesign/2019/dec/27/robin-welch-obituary, accessed 23 March 2021) Initially studying at Penzance School of Art under Michael Leach (son of Bernard Leach) and the Central School of Art, London Robin Welch then worked part-time at the Leach Pottery between 1953 and 1959 before opening his own pottery in London's west end (1960 to 1962). After a couple of years of world travel, including working in Australia from 1962 to1965 helping Ian Sprague set up his Mungeribar Pottery and exhibiting in Melbourne, Robin Welch returned to England setting up Stadbroke Pottery in Eye, Suffolk in 1965. Apart from his studion work Robnin Welch was a skilled designer for industry including Wedgwood. When not in his Suffolk studio Robin Welch spent much time in Australia where he appreciated the outback’s arid earth, brilliant light, grittier textures and luminous colour. Stoneware bowl on a tall foot. Calcium matt glaze, underglaze colour with underglaze metallic lustre. ceramic, jan feder memorial ceramics collection, robin welch, gippsland campus, mungeribar pottery, stadbroke pottery -
 Glenelg Shire Council Cultural Collection
Glenelg Shire Council Cultural CollectionDomestic object - Dish, n.d
Found at ship wreck site off Neve Valley by abalone diver in the early 1990s6 sided glass dish. Clear glass with pattern on sides and base. No major chips out of glass. Calcium deposits on one side.Front: - Back: -shipwreck, glass dish, diver -
 Flagstaff Hill Maritime Museum and Village
Flagstaff Hill Maritime Museum and VillageLamp
Lamp carbide metal with hinge, latch and thick glass lens. Part where calcium carbide is missing. Maker Reimann's, Phaenomen. Munichflagstaff hill, warrnambool, shipwrecked-coast, flagstaff-hill, flagstaff-hill-maritime-museum, maritime-museum, shipwreck-coast, flagstaff-hill-maritime-village -
 Glenelg Shire Council Cultural Collection
Glenelg Shire Council Cultural CollectionDomestic object - Glass Dish, n.d
Found at ship wreck site off Neve Valley by Abalone Diver some 10-15 years ago.Glass dish with pattern on sides and underside. Chipped on two corners. Scratched on most of the outside. Calcium deposits at one end. Rectangle shape. Glass tint slightly yellow.Front: - Back: - -
 Musculoskeletal Health Australia (now held by the Glen Eira Historical Society)
Musculoskeletal Health Australia (now held by the Glen Eira Historical Society)Photograph - Objects, May 2003
In May 2003, Arthritis Victoria (AV) and Osteoporosis Victoria (OV) had an information stand at the Women's Expo. The stand had fact sheets and nutritional information available for women and girls to take home, as well as examples of fresh and packaged food items suitable for a nutritional and calcium-rich diet. This photo depicts the AV/OV stand at the Women's Expo.Colour photo of an information stand at an expo. On a rectangular table covered with a red tablecloth, there is a range of printed factsheets arranged on display stands, and fresh and packaged food items with labels attached. At the top of the information booth, there is a sign that says, "ARTHRITIS & OSTEOPOROSIS VICTORIA". Behind the table, there are posters, brochures, and information sheets on a dividing wall separating the stalls. The text on the poster says, "OSTEOPOROSIS VICTORIA Are you at risk of Osteoporosis? FIND OUT HERE". There are also two blue deck chairs behind the table. Another booth is evident in the background.[On a white label, handwritten in black ink] stand at women's expo May 2003arthritis victoria, av, osteoporosis victoria, ov, arthritis foundation of victoria, afv, womens expo, information stand, information booth, information stall, factsheets, bone health, strong bones, healthy bones, calcium, 2003 -
 Flagstaff Hill Maritime Museum and Village
Flagstaff Hill Maritime Museum and VillageFunctional object - Carbide Lamp, Powell & Hanmer, 1920s
Francis Powell (1861-) and Francis Hanmer (1858-1925) founded Powell and Hanmer Ltd in the Summer of 1885 for the manufacturer of bike and carriage lamps. Their first advertisements began to appear in November of 1885. In 1890 they lodged a Patent for “velocipede” lamps to be used by lightweight wheeled vehicles propelled by a rider, such as a bike, tricycle and railroad handcar. In April of 1913, they were selling headlamps for cars and in 1914 built their second factory manufacturing dynamo lighting sets in Rocky Lane Birmingham, also for the production of dynamos for motor cars. Then in 1929 Powell and Hanmer Ltd, was acquired by the Lucas company which was at that time the main competitor for the manufacture of non-electrical equipment for cycles and motorcycles. When a director of Powell and Hanmer joined the board of Austin motor cars, Lucas feared that Austins might encourage Powell and Hanmer to start to produce electrical equipment for supply to the company and as a result this association might affect Lucas's business with other large vehicle manufacturers. As a result, Lucas made an offer to Powell & Hanmer and purchased the business for £500,000. Carbide lighting was used in rural and urban areas of Australia which were not served by electrification. Its use began shortly after 1900 in many countries and continued past the 1950s. Calcium carbide pellets were placed in a container outside the home, with water piped to the container and allowed to drip on the pellets releasing acetylene. This gas was piped to lighting fixtures inside the house, where it was burned, creating a very bright flame. Carbide lighting was inexpensive but was prone to gas leaks and explosions. Early models of the automobile, motorbike and bicycles used carbide lamps as headlamps. Acetylene gas, derived from carbide, enabled early automobiles to drive safely at night. Thick concave mirrors combined with magnifying lenses projected the acetylene flame light. These type of lights were used until reliable batteries and dynamos became available, and manufacturers switched to electric lights. Acetylene lamps were also used on riverboats for night navigation. The National Museum of Australia has a lamp made in about 1910 that was used onboard the PS Enterprise, an 1878 Australian paddle steamer, currently owned by the National Museum of Australia in Canberra. It is still operational, and one of the oldest working paddle steamers in the world, listed on the Australian Register of Historic Vehicles.Acetylene Carbide lamp, Model “Panther” distinct patterned side red and green lenses. These lamps were also known as acetylene gas lamps. They work off a chemical reaction between calcium carbide and water.Model 75flagstaff hill, warrnambool, flagstaff-hill-maritime-museum, maritime-museum, shipwreck-coast, flagstaff-hill-maritime-village, carbide lamp, motor vehicle, bike lamp, lighting, vehicle lighting, powell, hanmer, acetylene gas lamp, early lighting -
 Stawell Historical Society Inc
Stawell Historical Society IncRealia, Fossil's
Alan Tangey returned with them after working as a survey technician from Stawell area and given to children.Reputed to be Fossilised Eggs and 8 Pieces of wood, also 1 piece of Fossilsed Coral. Probably Fossilised Plant Roots Formed by Cementing Around Roots by minerals such as Calcium. Coral may have come from coastal survey work or holidays. fossils, children -
 The Beechworth Burke Museum
The Beechworth Burke MuseumGeological specimen - Actionlite and Pyrite
Actinolite is usually found in metamorphic rocks, such as contact aureoles surrounding cooled intrusive igneous rocks. It also occurs as a product of the metamorphism of magnesium-rich limestones. Pyrite is usually found with other sulfides or oxides in quartz veins, sedimentary rock, and metamorphic rock, as well coal beds, and as a replacement mineral in fossils. Actinolite is an amphibole silicate mineral. It is named after the Greek word "aktinos" meaning “ray” in allusion to the mineral's fibrous nature. Fibrous actinolite is a type of asbestos and was once mined along Jones Creek at Gundagai, New South Wales. Pyrite or "Fool's Gold" is the most common sulfide mineral. It is named after the Greek "pyr" meaning "fire" because it can be used to create sparks needed for a fire if struck against metal or a hard surface. Due to its gold colour, pyrite can be mistaken for gold and often forms alongside it, causing small amounts of gold to be present in rocks containing pyrite. Most importantly, pyrite is an ore of gold. Pyrite is sometimes used as a gemstone but is not great for jewellery as it easily tarnishes. In some fossils of ammonites – shelled cephalopods that died ~66 million years ago – pyrite also replaces the shell. This specimen is part of a larger collection of geological and mineral specimens collected from around Australia (and some parts of the world) and donated to the Burke Museum between 1868-1880. A large percentage of these specimens were collected in Victoria as part of the Geological Survey of Victoria that begun in 1852 (in response to the Gold Rush) to study and map the geology of Victoria. Collecting geological specimens was an important part of mapping and understanding the scientific makeup of the earth. Many of these specimens were sent to research and collecting organisations across Australia, including the Burke Museum, to educate and encourage further study. A small-medium-sized solid specimen with the minerals actinolite (dark green fibrous) and pyrite (brassy) with shades of brown, black/grey, and white. Actinolite is an amphibole mineral in the tremolite-actinolite series of calcium, magnesium, and iron silicates. Pyrite is an iron disulfide mineral.geological specimen, geology, geology collection, burke museum, beechworth, indigo shire, geological, mineralogy, pyrite, actinolite, victoria, sewyln, alfred selwyn -
 Musculoskeletal Health Australia (now held by the Glen Eira Historical Society)
Musculoskeletal Health Australia (now held by the Glen Eira Historical Society)Photograph - Group photo, 2002
In 2002, Arthritis Victoria (AV) presented an Osteoporosis Seminar. The seminar was held at AV's headquarters in Elsternwick. In this photo, an unidentified woman addresses the seminar attendees.COL photo of a woman speaking into a microphone at a lectern. Behind her is a projector screen displaying a slide image titled, "The Latest Views on Calcium & Healthy Eating". To the side, there is a large bouquet of flowers in a vase on a stand. On the wall, there is a poster with the image of two hands holding a wishbone, with the text "WORLD OSTEOPOROSIS DAY" below.arthritis foundation of victoria, afv, osteoporosis victoria, ov, arthritis victoria, av, seminar, presentation, elsternwick, 2002 -
 Musculoskeletal Health Australia (now held by the Glen Eira Historical Society)
Musculoskeletal Health Australia (now held by the Glen Eira Historical Society)Photograph - Group photo, Circa 1990-1991
Representatives from the Arthritis Foundation of Victoria (AFV), event partners and sponsors pose for a photo at the Osteoporosis Prevention and Self Management Course media launch, circa 1990-1991. This photo appears on page 6 of the 1991 Annual Report with the caption: "Enjoying high calcium refreshments at the Osteoporosis Prevention and Self Management Course media launch are Foundation President, Dr Leslie Koadlow; Rhonda Galbally, Chief Executive of VicHealth; Jan Ryan, Ministry of Health representative; Carmel Travers for Sandhurst Farms; Professor Ken Muirden; and Jenny Davidson, Foundation Education and Services Manager."B&W photo of a small group of people standing in a semi-circle in front of a large window and a freestanding vertical display board. They are each holding a disposable PhysiCal branded milk-shake cup with a straw. There is a large poster on one of the windows, which contains the text (partially obscured), "Promoting good health and preventing illness in our community". On one panel of the display board, there is the text, "Arthritis Foundation of Victoria 862 2555", and some large posters of people doing various types of exercises, including water exercise.arthritis foundation of victoria, afv, osteoporosis prevention and self management course, dr leslie koadlow, rheumatologist, president, rhonda galbally, chief executive, vichealth; jan ryan, ministry of health, representative, carmel travers, sandhurst farms, professor ken muirden, jenny davidson, education and services manager, physi-cal, calcium, bone health, annual report, c1990, c1991 -
 Kew Historical Society Inc
Kew Historical Society IncContainer - D.C.P. Wafers
D.C.P. Wafers were produced by Park, Davis & Co, Sydney. This U.S.-based company was started in Detroit in 1866. It was once the largest pharmaceutical company in the world. The active ingredient - Dibasic calcium phosphate. Small printed cardboard box, once containing wafers of D.C.P. Wafers, containers, park david & co -- sydney, d.c.p wafers -
 Federation University Historical Collection
Federation University Historical CollectionTool - Object, E. Thomas & Williams Limited, Cambrian Lampworks, Kop Staszic Carbide Lamp
Carbide lamps, or acetylene gas lamps, are simple lamps that produce and burn acetylene (C2H2) which is created by the reaction of calcium carbide (CaC2) with water. Acetylene gas lamps were used to illuminate buildings, as lighthouse beacons, and as headlights on motor-cars and bicycles. Portable acetylene gas lamps, worn on the hat or carried by hand, were widely used in mining in the early twentieth century. A mining or caving lamp has calcium carbide placed in a lower chamber, the generator. The upper reservoir is then filled with water. A threaded valve or other mechanism is used to control the rate at which the water is allowed to drip into the chamber containing the calcium carbide. By controlling the rate of water flow, the production of acetylene gas is controlled. This, in turn, controls the flow rate of the gas and the size of the flame at the burner, and thus the amount of light it produces. Staszic is a coal mine located in Katowice , in the district Giszowiec, Poland This type of lamp generally has a reflector behind the flame to help project the light forward. An acetylene gas powered lamp produces a bright, broad light. Many cavers prefer this type of unfocused light as it improves peripheral vision in the complete dark. The reaction of carbide with water produces a fair amount of heat independent of the flame. In cold caves, carbide lamp users can use this heat to help stave off hypothermia. When all of the carbide in a lamp has been reacted, the carbide chamber contains a wet paste of slaked lime (calcium hydroxide). This is emptied into a waste bag and the chamber can be refilled. (http://en.wikipedia.org/wiki/Carbide_lamp, accessed 06/05/2015)Metal Carbide Mining lamp with light reflector and hook.miners lamp, mining lamp, statszic, poland, carbide, mining -
 Flagstaff Hill Maritime Museum and Village
Flagstaff Hill Maritime Museum and VillageTextile - Flour bag - McAlpin's Flour containing Aerophos'
Flour bags were used when purchasing flour from the late 19th century to the mid 20th century. In Melbourne, McAlpin's were one of the best known suppliers of self-raising flour. The business first began as a bakery in 1879 and was taken over in 1959. This is a good example of a flour bag from a well know Victorian supplier prominent in the early twentieth century.Calico flour bag for McAlpin’s self-raising flour. The stencil on the bag is very faded especially where red ink has been used. It includes an image of mountains and fir trees in green ink and a stalk of wheat sweeping up from bottom right to top / middle left in red ink.McAlpin’s (faded red) CONTAINING AEROPHOS’, PHOSPHATE AERATOR, SELF-RAISING, FLOUR (faded red), “THERE’S NO OTHER” Faded red square with writing …. CALCIUM …. Faded red triangle with T inside it to the far right of the trees Faded red writing at the bottom of the bag: PREPARED WITH PHOSPHATE AERATOR, AEROPHOS 25 LBS NET flagstaff hill maritime museum and village, great ocean road, shipwreck coast, calico, domestic object, textile, food storage, flour bag, mcalpin's, self-raising flour -
 The Beechworth Burke Museum
The Beechworth Burke MuseumGeological specimen - Loellingite in Rhodonite
Rhodonite is a reddish-pink manganese silicate material and often contains iron, magnesium and calcium. It is usually found in metamorphic rocks (rocks which have been altered by heat, pressure or chemical process). It can range in size from tiny to massive. Because of their composition they are not suitable for use in jewellery because they are hard enough. It is quite rare to find, though has been found in Australia, North America, South America and Europe.This specimen is part of a larger collection of geological and mineral specimens collected from around Australia (and some parts of the world) and donated to the Burke Museum between 1868-1880. A large percentage of these specimens were collected in Victoria as part of the Geological Survey of Victoria that begun in 1852 (in response to the Gold Rush) to study and map the geology of Victoria. Collecting geological specimens was an important part of mapping and understanding the scientific makeup of the earth. Many of these specimens were sent to research and collecting organisations across Australia, including the Burke Museum, to educate and encourage further study. This specimen is a palm shaped piece of loellingite within rhodonite. It is mostly reddish-pink coloured, with flecks of light and dark grey. Loellingite is a grey iron arsenide which often forms into crystal shapes. It is mostly found in mesothermal veins (caused by immense heat) with sulfides or in limestone. It is toxic when heated or struck. rhodonite, loellingite, burke museum, beechworth, geological survey of victoria -
 Parks Victoria - Days Mill and Farm
Parks Victoria - Days Mill and FarmFurniture - Linoleum Sheeting, C 1900
This linoleum is in-situ in one of the rooms of the house at Days Mill. Newspaper laid underneath indicates the linoleum was laid after September 1910.PATTERNED LINOLEUM : A floor covering made from materials such as solidified linseed oil (linoxyn), pine rosin, ground cork dust, wood flour, and mineral fillers such as calcium carbonate, most commonly on a burlap or canvas backing. This piece has a cream & light grey mosaic type background with circular brown leaf repeating patterns interlaced into four square blue shapes. Central star pattern within circular leaf pattern in light & dark blues & browns. Stylised floral motif in between each circular & square shaped pattern repeats. -
 Orbost & District Historical Society
Orbost & District Historical Societycoach lamps, 1920's
These lamps belonged to Ernie Eaton and were used for spotlighting rabbits. Carbide lamps, or acetylene gas lamps, are simple lamps that produce and burn acetylene (C2H2) which is created by the reaction of calcium carbide (CaC2) with water. Acetylene gas lamps were used to illuminate buildings, as lighthouse beacons, and as headlights on motor-cars and bicycles. Portable acetylene gas lamps, worn on the hat or carried by hand, were widely used in mining in the early twentieth century. They are still employed by cavers, hunters, and cataphiles Torches, candles, oil lamps and kerosene lamps were designed to be carried around but they could be dangerous because they have flame as a source of light. These lanterns are significant examples of lighting devices widely used used before the use of battery powered devices. A pair of Germania lamps. They have brass cases with steel bodied generators and convex lens. .1 is a metal carbide coach lamp. .2 is a similar lamp but has the chimney missing. .3 is a metal handle used to attach a lamp to the front of the vehicle.Germania Base has circle with three leaves.lantern lamp germania coach-lamp -
 Musculoskeletal Health Australia (now held by the Glen Eira Historical Society)
Musculoskeletal Health Australia (now held by the Glen Eira Historical Society)Photograph - Group photo, Circa 1990-1991
Two unidentified people pose for a photo at the Osteoporosis Prevention and Self Management Course media launch, circa 1990-1991.B&W medium close up photo of a man and a woman posing for a photo. They are each holding a PhysiCal branded disposable milkshake cup with a straw. The woman is also holding an hors d'oeuvre in her other hand. In the background, there are several other people standing and chatting. One woman is drinking from a cup. There are some posters on the windows, beyond which some wooden lattice panels are evident around the outside of the windows.arthritis foundation of victoria, afv, osteoporosis prevention and self management course, physi-cal, calcium, bone health, c1990, c1991 -
 City of Moorabbin Historical Society (Operating the Box Cottage Museum)
City of Moorabbin Historical Society (Operating the Box Cottage Museum)Jewellery, pearl necklace, 20thC
It is thought that natural pearls form under a set of accidental conditions when a microscopic intruder or parasite enters a bivalve mollusc and settles inside the shell. The mollusc, irritated by the intruder, forms a pearl sac of external mantle tissue cells and secretes the calcium carbonate and conchiolin to cover the irritant. This secretion process is repeated many times, thus producing a pearl. Natural pearls come in many shapes, with perfectly round ones being comparatively rare. In general, cultured pearls are less valuable than natural pearls, whereas imitation pearls have almost no value. Cultured freshwater pearls can often be confused for natural pearls Cultured pearls are the response of the shell to a tissue implant. A tiny piece of mantle tissue (called a graft) from a donor shell is transplanted into a recipient shell, causing a pearl sac to form into which the tissue precipitates calcium carbonate. Some imitation pearls (also called shell pearls) are simply made of mother-of-pearl, coral or conch shell A strand of pearls called a princess length, 43 to 48 cm in length, comes down to or just below the collarbone. A graduated strand of pearls most often has at least 3 mm of differentiation from the ends to the centre of the necklace. A lady's pearl necklace and 1 earring in a hinged, lined, cream Bakelite case .jewellery, necklace, earring, pearls, market gardners, early settlers, moorabbin, bentleigh, cheltenham, ormond -
 Department of Energy, Environment and Climate Action
Department of Energy, Environment and Climate ActionSpeedy Moisture Meter, Thomas Ashworth and Co, c 1950
The most common technique to measure fuel moisture content in Victorian forests until recently was the Speedy Moisture Meter - which is essentially a pressure cylinder. Originally developed in England during the 1920s for measuring moisture in wheat and other grains it was adapted for Australian forest fuels in the 1950s. Fuel was first ground using a Spong domestic food mincer, often attached to the bullbar of a vehicle, and a small measured (using the beam balance) sample placed into the Speedy together with a measured amount of calcium carbide and then sealed. The chemical reaction between the moisture in the fuel and the calcium carbide created acetylene gas; the pressure of which was read on the external dial which gave the read-out as the moisture content (percentage of net weight) of the sample. There were important techniques with preparing the minced samples, using the chemical and the subsequent cleaning of the Speedy to give reliable readings, but it was quick, inexpensive, robust, portable and practical in the field - it gave readings which were reproducible to within +/- 0.5% moisture content. It was used routinely before igniting a fuel reduction burn or measuring fuel moisture differentials on slash burns. In about 1996, Karen Chatto and Kevin Tolhurst from the Department’s Creswick Research Station developed the Wiltronics Fuel Moisture meter which measured electrical resistance.First reliable tool for measuring bushfire fuel moisture content in the fieldSpeedy Moisture Meter in wooden boxmanufacture's marks and instructions on usebushfire, forests commission victoria (fcv) -
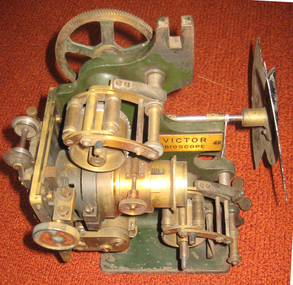 Federation University Historical Collection
Federation University Historical CollectionEquipment - Projector, Victor Bioscope, c1910
A Bioscope show was a fairground attraction consisting of a travelling cinema. The heyday of the Bioscope was from the late 1890s until World War I. Bioscope shows were fronted by the largest fairground organs, and these formed the entire public face of the show . A stage was usually in front of the organ, and dancing girls would entertain the crowds between film shows. Films shown in the Bioscope were primitive, and the earliest of these were made by the showmen themselves. Later, films were commercially produced. Bioscope shows were integrated, in Britain at least, into the Variety shows in the huge Music Halls which were built at the end of the nineteenth century. After the Music Hall Strike of 1907 in London, bioscope operators set up a trade union to represent them. There were about seventy operators in London at this point. (http://en.wikipedia.org/wiki/Bioscope_show) The Projector was a rather unreliable piece of apparatus, powered by a variety of light sources, including Calcium Oxide (Lime-Light). A Calcium Carbide Burner, or the rather more superior Carbon Arc. All these methods were highly unpredictable & quite frankly...dangerous! Often resulting in explosions, burning down the entire Show! (which is probably why NO original Shows still exist. Alfred Ball's Bioscope, pictured below, built in 1905 was struck by lightning, shortly after the picture was taken! (http://www.circus-entertainer.co.uk/heritage.htm) In 1909 the first bioscopes pictures were shown at the Ballaarat Mechanics' Institute.Brass and green painted metal film projectorbioscope, vector, entertainment, projector, film, theatre, movie -
 Musculoskeletal Health Australia (now held by the Glen Eira Historical Society)
Musculoskeletal Health Australia (now held by the Glen Eira Historical Society)Photograph - Solo photo, Circa 1990-1991
A young girl drinks a milkshake from a PhysiCal branded disposable milkshake cup. There are other people standing behind her. They appear to be in a paved outdoor retail shopping precinct.B&W medium close up photo of a young girl drinking through a straw from a PhysiCal branded disposable milkshake cup. She is standing outdoors on a paved brick floor. There is a crowd of people behind her. Through a gap in the crowd, some shops are evident in the distance.arthritis foundation of victoria, afv, national arthritis week, naw, bourke st mall, osteoporosis prevention, physi-cal, calcium, bone health, 1991 -
 Bendigo Historical Society Inc.
Bendigo Historical Society Inc.Book - BILL ASHMAN COLLECTION: NOTE BOOK
Green, indexed, cloth bound note book containing fourteen pages of hand-written scientific experiments. Subjects include Iron, Standardization of KMn O4 solution, Ferrous Ammonium Sulphate, Sodium Oxalate?, Assay of Titaniferous? Iron ore, To make up Standard solution of Na2 S2 O? 5? For Copper, Standard Sol of (?H4)2 Mo O4) for Lead Assay, Sry Assay of Lead, Antimony, Resolution of ? Compounds, Clarks Modified Method Ores & Alloys, Method of reducing antimony solutions, Bromate Method, Standard Sol Pot Bromate, Standard, Assay, Oxides, Method suitable for alloys of Pb ? ?, Arsenic, Standard Iodine, Starch, Assay, Tungsten, Assay for Pyritic Scheelite, Illuminating Gas, Calcium, Permanganate method, Assay, ? O2, Iron, Ca O, Norman Solutions.sciences, instruments-general, scalebuoy, bill ashman collection, scientific formulas, assay
