Showing 14 items matching "iud"
-
 Royal Australian and New Zealand College of Obstetricians & Gynaecologists (RANZCOG)
Royal Australian and New Zealand College of Obstetricians & Gynaecologists (RANZCOG)Equipment - Lippes Loop IUD associated with Dr Lachlan Hardy-Wilson, Ortho Pharmaceutical Corporation
Developed by Dr Jack Lippes in 1962, the Lippes Loop was commonly used as a contraceptive device from the 1960s to the 1980s. Due to its low cost and the ease of inserting and removing the device, it quickly became the most popular IUD in the world during its time. This is one of a collection of items received from the practice of Dr Lachlan Hardy-Wilson, FRCOG, Launceston, Tasmania.Lippes Loop IUD, Size B. IUD and inserter are sealed inside a sterile plastic pocket. Manufacturer information is printed on a cardboard insert which holds the IUD inside the pocket. The packaging of the IUD is printed with instructions for use on the back.intrauterine device -
 Royal Australian and New Zealand College of Obstetricians & Gynaecologists (RANZCOG)
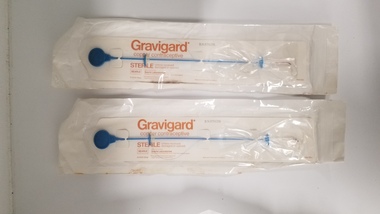
Royal Australian and New Zealand College of Obstetricians & Gynaecologists (RANZCOG)Equipment - Two Gravigard IUDs associated with Dr Lachlan Hardy-Wilson, Searle Laboratories
Copper intrauterine devices (IUDs), first marketed in the early 1970s, represent an important contraceptive option for 150 million women worldwide. The method is safe, rapidly reversible, inexpensive, highly effective, long-acting (up to 20 years for some products [1]), and non-hormonal; these attributes make it unique and desirable for many users. However, increased bleeding and pain cause up to 15% of users to have the device removed within the first year [2]; still higher percentages tolerate some level of these side effects yet retain use of the method. In one study, 67% of women using the TCu380A complained about menstrual side effects within the first year of use [3]. (Hubacher et al, 'Side effects from the copper IUD: do they decrease over time?', 2009)This is one of a collection of items received from the practice of Dr Lachlan Hardy-Wilson, FRCOG, Launceston, Tasmania.Two sealed Gravigard IUDs. IUD and inserter are sealed inside a sterile plastic pocket. Manufacturer information is printed on a cardboard insert which holds each IUD inside the pocket.intrauterine device -
 Royal Australian and New Zealand College of Obstetricians & Gynaecologists (RANZCOG)
Royal Australian and New Zealand College of Obstetricians & Gynaecologists (RANZCOG)Equipment - Two Lippes Loop IUDs associated with Dr Lachlan Hardy-Wilson, Ortho Pharmaceutical Corporation
Developed by Dr Jack Lippes in 1962, the Lippes Loop was commonly used as a contraceptive device from the 1960s to the 1980s. Due to its low cost and the ease of inserting and removing the device, it quickly became the most popular IUD in the world during its time. This is one of a collection of items received from the practice of Dr Lachlan Hardy-Wilson, FRCOG, Launceston, Tasmania.Two sealed Lippes Loop IUDs, Size C. IUD and inserter are sealed inside a sterile plastic pocket. Manufacturer information is printed on a cardboard insert which holds each IUD inside the pocket. The packaging of the first Lippes Loop is printed with an expiry date of Jan 83 on the back. The packaging of the second Lippes Loop is printed with instructions for use on the back.l intrauterine device -
 Royal Australian and New Zealand College of Obstetricians & Gynaecologists (RANZCOG)
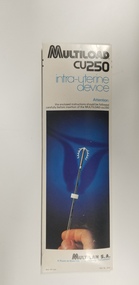
Royal Australian and New Zealand College of Obstetricians & Gynaecologists (RANZCOG)Equipment - Multiload CU250 IUDs associated with Dr Lachlan Hardy-Wilson, Multilan S.A, 1984
Multiload is an IUD, an intrauterine device used for contraception. The plastic used in this IUD is a mixture of high density polyethylene, ethylene vinyl acetate copolymer and barium sulphate in a weight ratio 44/36/20. (MIMS, October 2013)This is one of a collection of items received from the practice of Dr Lachlan Hardy-Wilson, FRCOG, Launceston, Tasmania.Two flat boxes containing Multiload CU250 intrauterine devices. Front of box carries an image of a hand holding an IUD inserter, with the Multiload IUD at the top of the inserter, above a blue background on which an image of a uterus has been superimposed. Text printed on the front of the box reads 'MULTILOAD/CU250/intra-uterine/device/Attention:/the enclosed instructions should be followed carefully before insertion of the MULTILOAD-cu250'. Manufacturer's information is printed on the bottom section of the front of the box. Back of box is printed with distributor information. One box is sealed, and the other box has been opened. Back of sealed box carries a sticker which reads 'Date of manufacture: /12-08-1984'. Opened box contains a Multiload IUD in a sealed packet, a brochure outlining the recommended procedure for insertion, a booklet outlining prescribing information, and another booklet entitled 'Everything you have to know about Multiload'. The packaging of the IUD inside the opened box is labelled with an expiry date of 16/10/1984.intrauterine device -
 Royal Australian and New Zealand College of Obstetricians & Gynaecologists (RANZCOG)
Royal Australian and New Zealand College of Obstetricians & Gynaecologists (RANZCOG)Equipment - Multiload CU250 IUDs associated with Dr Lachlan Hardy-Wilson, Multilan S.A, 1984
Multiload is an IUD, an intrauterine device used for contraception. The plastic used in this IUD is a mixture of high density polyethylene, ethylene vinyl acetate copolymer and barium sulphate in a weight ratio 44/36/20. (MIMS, October 2013)This is one of a collection of items received from the practice of Dr Lachlan Hardy-Wilson, FRCOG, Launceston, Tasmania.Two flat boxes containing Multiload CU250 intrauterine devices. Front of each box carries an image of a hand holding an IUD inserter, with the Multiload IUD at the top of the inserter, above a blue background on which an image of a vagina has been superimposed. Text printed on the front of the box reads 'MULTILOAD/CU250/intra-uterine/device/Attention:/the enclosed instructions should be followed carefully before insertion of the MULTILOAD-cu250'. Manufacturer's information is printed on the bottom section of the front of the box. Back of each box is printed with distributor information. One box is sealed, and one box is unsealed. Unsealed box contains one IUD, sealed inside a sterile plastic pocket. Back of sterile pocket is printed with an expiration date of 06-12-1984. The IUD is in the form of a small plastic rod, or stem, with two small flexible side-arms. Each side arm has five small protrusions. A copper wire is wound around the stem. A nylon thread with two ends is attached to the bottom end of the stem. Back of sealed box carries a sticker which reads 'Date of manufacture: /12-08-1984'.intrauterine device -
 Royal Australian and New Zealand College of Obstetricians & Gynaecologists (RANZCOG)
Royal Australian and New Zealand College of Obstetricians & Gynaecologists (RANZCOG)Equipment - Gravigard IUD associated with Dr Lachlan Hardy-Wilson, Searle Laboratories
Copper intrauterine devices (IUDs), first marketed in the early 1970s, represent an important contraceptive option for 150 million women worldwide. The method is safe, rapidly reversible, inexpensive, highly effective, long-acting (up to 20 years for some products [1]), and non-hormonal; these attributes make it unique and desirable for many users. However, increased bleeding and pain cause up to 15% of users to have the device removed within the first year [2]; still higher percentages tolerate some level of these side effects yet retain use of the method. In one study, 67% of women using the TCu380A complained about menstrual side effects within the first year of use [3]. (Hubacher et al, 'Side effects from the copper IUD: do they decrease over time?', 2009)This is one of a collection of items received from the practice of Dr Lachlan Hardy-Wilson, FRCOG, Launceston, Tasmania.Sealed Gravigard IUD in original box. IUD and inserter are sealed inside a sterile plastic pocket. The box is made of cardboard and has an orange top panel with white sides. Text printed on the front of the box reads '1 Sterile Pack/Gravigard*/contraceptive/copper/contraceptive/FRAGILE/Do not bend or crush/SEARLE'. Text printed on side of box reads 'Made in England Supplied by Searle Laboratories, Division of Searle Australia Pty. Ltd., Sydney, Australia'.intrauterine device -
 Royal Australian and New Zealand College of Obstetricians & Gynaecologists (RANZCOG)
Royal Australian and New Zealand College of Obstetricians & Gynaecologists (RANZCOG)Inhiband (Hall) intrauterine devices with dispensing box, associated with Professor Geoff Bishop, Ayerst International Inc, 1960s-early 1970s
The Inhiband intrauterine device is a variety of the Grafenberg Ring. The Grafenberg ring was developed by Dr Ernst Grafenberg in the late 1920s. This coincided with the beginnings of the modern birth control movement. Grafenberg and Herbert Hall migrated to the USA during the Hitler era and brought with them the knowledge of the intrauterine ring. Herbert Hall developed a stainless steel version of the Grafenberg ring in 1949 and used it with select private patients in New York. A report on his results was published in the American Journal of Obstetrics and Gynecology in 1962. The Inhiband product bears his name in brackets. The dispensing box and five remaining containers with Inhiband IUDs inside were from the Albert Street East Melbourne rooms of Dr Geoffrey Bishop. This contraceptive device was commonly used in the 1960s-early 1970s.White plastic container with clear plastic hinged lid and white plastic insert with slots for 10 individual containers of Inhiband IUDs. Contains five individual white plastic containers which hold Inhiband IUDs. The five intrauterine devices resemble a metal ring in design and are unused and still in their packaging. contraceptive, intrauterine device -
 Royal Australian and New Zealand College of Obstetricians & Gynaecologists (RANZCOG)
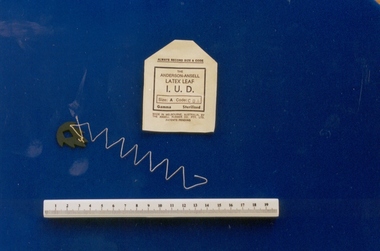
Royal Australian and New Zealand College of Obstetricians & Gynaecologists (RANZCOG)Intrauterine device, Anderson-Ansell design, Ansell, 1970s
The only IUD of Australian origin, developed in 1971 by Australian doctor Ian Anderson in partnership with Ansell Australia and produced at the Ansell factory, 18 River Street, Richmond in the 1970s. The device was taken up by the Battelle Foundation, USA, and clinical trials were carried out in Israel, Indonesia and Singapore. No formal clinical trials were carried out in Australia. The device was used extensively by Population Services International, a private abortion clinic in Sydney in the mid 1970s. At the time this device was donated to the collection in 1997, there were legal proceedings regarding two Australian women who claimed ongoing problems as a result of its use. The donor, Dr Richard Gutch, practised in Clifton Hill and used some in the 1970s but not for long. The drawbacks were the multi-filament string acted as a wick for infection, also the latex leaf often came away from the thread when pulled for removal. Curettage was often the only effective way to remove the IUD. Dr Gutch removed many as he developed a reputation for being skilled at their removal.Intrauterine device (IUD), Ansell-Anderson latex leaf design, with paper sleeve. Made of latex impregnated with copper and zinc. The IUD is leaf-shaped with serrated edge and diamond shape cut out from its centre. Small hole in tail with attached twisted cream coloured thread. Paper sleeve is stamped, "THE/ ANDERSON-ANSELL/LATEX LEAF/I.U.D". SIZE:A/PATENTS PENDING", and notes it is Size A.contraceptive, intrauterine device -
 Royal Australian and New Zealand College of Obstetricians & Gynaecologists (RANZCOG)
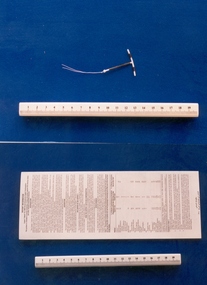
Royal Australian and New Zealand College of Obstetricians & Gynaecologists (RANZCOG)Intrauterine device, Copper T model TU380A
The TCu380A IUD is the most widely used copper IUD in the world. Although it is claimed that the TCu380A IUD has been shown to be an effective, safe, long-term contraceptive device, a recent study has noted that it causes side effects and early removal for many users.Intrauterine device. Copper T model TU380A, made of copper and white plastic. T-shaped, with two threads of cotton attached. Includes patient information leaflet [23.2].contraception, intrauterine device -
 Royal Australian and New Zealand College of Obstetricians & Gynaecologists (RANZCOG)
Royal Australian and New Zealand College of Obstetricians & Gynaecologists (RANZCOG)Dispensing box, Inhiband (Hall) intrauterine device, Ayerst International Inc, 1960s-early 1970s
These items came from the Albert Street East Melbourne rooms of Dr Geoffrey Bishop. The contraceptive device was commonly used in the 1960s-early 1970s.This is a variety of the Grafenberg Ring. The Grafenberg ring was developed by Dr Ernst Grafenberg in the late 1920s This coincided with the beginning of the modern birth control movement. Grafenberg and Herbert Hall et al migrated to the USA during the Hitler era and brought with them knowledge of the interuterine ring. Herbert Hall developed a stainless steel version of the Grafenberg ring in 1949 and used it with select private patients in New York. A report of his results in the American Journal of O&G in 1962. The Inhiband product bears his name in brackets. Container, plastic, containing Inhiband (Hall) intrauterine device in a sealed clear plastic bag [registration 30.3].contraceptive, iud -
 Royal Australian and New Zealand College of Obstetricians & Gynaecologists (RANZCOG)
Royal Australian and New Zealand College of Obstetricians & Gynaecologists (RANZCOG)Saf-T Coil intrauterine device associated with Dr Ronald McKenzie Rome, Deseret Pharmaceutical Co. Inc, c. 1965 - 1982
The “Saf-T-Coil” (Julius Schmid Laboratories, Little Falls, New Jersey, USA) IUD was a first generation IUD that entered manufacturing in 1965 and was one of the first inert type plastic IUDs to be commonly used. It was also one of the first to adopt the familiar T-shape of IUDs still used today. It was accepted during its time as being generally safe, effective and easy to insert, with low expulsion rates due to its bulky frame. Production was halted in 1982 for economic reasons as newer contraceptive methods gained popularity. (Madden et al. 'A Case of Migrating “Saf-T-Coil” Presenting With a Vesicovaginal Fistula and Vesicovaginal Calculus', https://www.sciencedirect.com/science/article/pii/S2214442016300286)Saf.T. Coil 33.s intrauterine device, with inserter. Sealed in original sealed plastic packaging, unopened. Shape resembles a double coil with ends that spiral inwards. The inserter has an adjustable blue 'stop' pre-set at 1 3/4" for insertion into a 'normal' uterus. There is also an instruciton leaflet enclosed.intrauterine device, contraception -
 Royal Australian and New Zealand College of Obstetricians & Gynaecologists (RANZCOG)
Royal Australian and New Zealand College of Obstetricians & Gynaecologists (RANZCOG)Equipment - Mark-7 disposable uterine sound associated with Dr Lachlan Hardy-Wilson, Searle Laboratories, 1984
Used to measure the depth/distance of the uterus. Uterine sounds are particularly useful for ensuring safe and accurate IUD placement.This is one of a collection of items received from the practice of Dr Lachlan Hardy-Wilson, FRCOG, Launceston, Tasmania.Disposable uterine sound in sterile sealed packaging. Label inside packaging reads 'Mark-7 brand of disposable uterine sound/ONE STERILE UNIT/NO. 154'. Label also includes manufacturer information, a sterile packaging warning, and a caution about sale of the item being restricted to physicians. The number '026' is printed on the back of the packaging.intrauterine device -
 Royal Australian and New Zealand College of Obstetricians & Gynaecologists (RANZCOG)
Royal Australian and New Zealand College of Obstetricians & Gynaecologists (RANZCOG)Lippes Loop intrauterine devices with container associated with Professor Geoff Bishop, Ortho Pharmaceutical Corporation
This item came from Geoff Bishop's rooms at Mollison House, 386 Albert Street, East Melbourne. Distributed by Ethnor P/L Sydney, these bulk purchase packs pre-dated the individually sterile packaged products with disposable applicators.Two white, plastic Lippes Loop IUDs [.1 and .2], with yellow string attached to one end of each. Double S-shape intrauterine devices. Also includes "LIPPES LOOP" product information label [.3], and a clear hinged plastic case with adhesive sticker [.4].intrauterine device, contraception -
 Royal Australian and New Zealand College of Obstetricians & Gynaecologists (RANZCOG)
Royal Australian and New Zealand College of Obstetricians & Gynaecologists (RANZCOG)Ten Lippes Loop intrauterine devices, with container, associated with Professor Geoff Bishop, Ortho Pharmaceutical Corporation
This item came from Geoff Bishop's rooms at Mollison House, 386 Albert Street, East Melbourne. Distributed by Ethnor P/L Sydney, these bulk purchase packs pre-dated the individually sterile packaged products with disposable applicators.Ten Lippes Loop IUDs [29.1-29.10], Size B. Made of white plastic with black threads attached at one end of each. Double S shape intrauterine device. With clear plastic bag [.11], clear plastic hinged container [.12] and paper product information sheet [.13].intrauterine device, contraception
