Showing 253 items
matching in c major
-
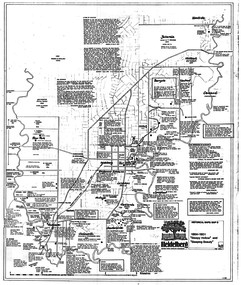 Greensborough Historical Society
Greensborough Historical SocietyMap, Heidelberg: Historic River Landscape Assessment. Historical maps. Map D 1864 -1901 "Sleepy Hollow" and "Sleeping beauty", 1985c
Geographical area of c. 35 sq. kilometres marked with physical features such as major roads, rivers, vegetation and properties, with locations and notes on the increasing number of farms, orchards and vineyards that had been developed in addition to pastoral runs. Bounded by Shire of Diamond Valley to the North, Darebin Creek to the West, and Plenty River to the East.Black and white sketch on white paper. Second copy 600 x 430 cm printed in black on semi-opaque paperheidelberg, heidelberg - maps -
 Greensborough Historical Society
Greensborough Historical SocietyDiagram, C. R. B. Traffic Census 1955-1960, 07/06/1960
The Country Roads Board, which had responsibility for major metropolitan roads, conducted a census of maximum hourly and daily traffic volumes recorded in 1955 and 1960 on 10 major roads in the Heidelberg and Greensborough areas.Black and white table G.M.H.traffic, heidelberg, greensborough, country roads board -
 Greensborough Historical Society
Greensborough Historical SocietyPhotograph - Digital Image, Katherine Rose Beale, 1840c
Photograph of a painting of Katherine Rose Beale (1795-1856), wife of Major Anthony Beale. The Beale family arrived in Port Phillip in 1839 and c.1841 established an estate named "St Helena" to the north of Melbourne. Katherine is buried in the St Katherine's Anglican Church Cemetery in Greensborough. The Church and its "Rose Chapel" were named after Katherine Rose Beale.Digital copy of colour photograph of a painting.st katherines church st helena, katherine rose beale, anthony beale -
 Glenelg Shire Council Cultural Collection
Glenelg Shire Council Cultural CollectionPrint, J. Macfarlane et al, Meeting of Major Mitchell and Edward Henty, Portland Bay, 1836, n.d
Displayed at History House.Lithograph, engraving of men on horses standing outside a wooden cottage and pole fence. Trees in background. On far left man is standing observing another standing man shaking hands with a central mounted figure. Framed under glass in wooden carved frame with inner gold-coloured frame.Front: Meeting of Major Mitchell and Edward Henty, Portland Bay, 1836 (engraved, lower centre -
 Greensborough Historical Society
Greensborough Historical SocietyArticle - Article, Journal, Women's World, Historic little church at St Helena, and the woman whom it commemorates; by C. K-H, 01/07/1934
The original Rose Chapel was erected in 1866 by Major Anthony Beale in memory of his wife Katherine Rose Beale; later renovated and handed over to the Church of England and renamed St Katherine's.Concerns the early history of St Helena and the Beale familyTwo photocopied pages, text with photos. 2 copiesWomen's World, 1/7/1934beale family, st katherines church st helena -
 4th/19th Prince of Wales's Light Horse Regiment Unit History Room
4th/19th Prince of Wales's Light Horse Regiment Unit History RoomTunic, Cotton, Paramount Mfg Co, 1941
Tunic is used to represent a tunic used in the Boer War 1899-1902. Although manufactured almost 40 years after this war ended, the style is almost identical to that used by VMR soldiers serving in the Boer War. The major difference is the stand and fall collar. In the Boer War, the collar was a stand up collar. A minor difference is the shape of the pocket flaps which on a tunic from the Boer War, was more rounded on the lower edge. The buttons are from a later period but were the closest available at the time the Tunic was assembled by the owner c.1994.Tunic, Khaki Cotton Drill, @ breast pockets with pleats. stand and fall collar, Inverted chevron, cuffs, Patrol back. AMF buttons, brass 7 total VMR shoulder titles, brass, 2 totalParamount Mfg Co 1941 size Regitmental No.... NAME..........tunic boer war mounted -
 Warrnambool RSL Sub Branch
Warrnambool RSL Sub BranchArmy Uniform trousers, Polyester Trousers, 1989
This item of uniform was own, worn and donated by Major (Retd) Bernard Farley during service in the Army Reserve Infantry at 8/7 Royal Victoria Regiment (RVR) Ballarat VICPolyester trousers with two side pockets, one back pocket, money pocket with belt loops and brass ceremonial belt keepersTag - A.D.I. P/L VIC 1989 ARROW indicating Govt. NSN 8405-66-0185-5522 MACQUARIE 89, Batch C Size. 80RF W.90 L.77 NAME. SERVICE NO. DRY CLEAN ONLY(A) Press on original creases. Use damp cloth. Medium to hot steam iron -
 Eltham District Historical Society Inc
Eltham District Historical Society IncPhotograph, Shire of Eltham Centenary Dinner, 6 Apr 1971
Cr. G. C. Dreverman presenting a silver centenary medal to His Excellency Major General Sir Rohan Delacombe, Governor of Victoria at the Shire of Eltham Centenary Dinner in 1971. The event was held in the West Riding Hall, Petrie Park Community Centre, Montmorency. This photo forms part of a collection of photographs gathered by the Shire of Eltham for their centenary project book, "Pioneers and Painters: 100 years of the Shire of Eltham" by Alan Marshall (1971). The collection of over 500 images is held in partnership between Eltham District Historical Society and Yarra Plenty Regional Library (Eltham Library) and is now formally known as 'The Shire of Eltham Pioneers Photograph Collection.' It is significant in being the first community sourced collection representing the places and people of the Shire's first one hundred years.Digital imagesepp, shire of eltham pioneers photograph collection, shire of eltham, shire of eltham centenary, official dinner, centenary celebrations, cr. g.c. dreverman, sir rohan delacombe -
 Eltham District Historical Society Inc
Eltham District Historical Society IncPhotograph, Shire of Eltham Centenary Dinner, 6 Apr 1971
His Excellency Major General Sir Rohan Delacombe, Governor of Victoria at the Shire of Eltham Centenary Dinner in 1971 with Cr. G. C. Dreverman, Cr Glover and Mrs Glover. The Premier, Mr Hamer can be seen in the background. The event was held in the West Riding Hall, Petrie Park Community Centre, Montmorency. This photo forms part of a collection of photographs gathered by the Shire of Eltham for their centenary project book, "Pioneers and Painters: 100 years of the Shire of Eltham" by Alan Marshall (1971). The collection of over 500 images is held in partnership between Eltham District Historical Society and Yarra Plenty Regional Library (Eltham Library) and is now formally known as 'The Shire of Eltham Pioneers Photograph Collection.' It is significant in being the first community sourced collection representing the places and people of the Shire's first one hundred years.Digital imagesepp, shire of eltham pioneers photograph collection, shire of eltham, shire of eltham centenary, official dinner, centenary celebrations, cr. g.c. dreverman, sir rohan delacombe, cr. glover, mrs. glover -
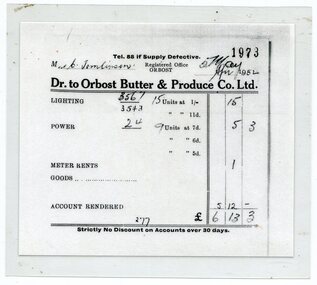 Orbost & District Historical Society
Orbost & District Historical SocietyDocument - invoice, Orbost Butter & Produce Co Ltd, 2 May 1952
In 1893, a group of Orbost farmers formed together to initiate the Orbost Butter Factory. The first factory was built in Lochiel Street and was a wooden building. In 1916, a new building was constructed on Forest Road. This was a more substantial brick building which had its own power source. Electric power, mainly for lighting was supplied to the developing township of Orbost by the Butter Factory. This docket is an invoice for the supply of power to the household or business of C. Tomlinson in 1952. The Butter Factory power was the only power to Orbost prior to the 1962 when S E C power came to Orbost. An invoice from a major business in Orbost which supplied power to the town for the first 50 years of the 20th Century. A laminated copy of an invoice from Orbost Butter & Produce Co Ltd. Black print on white paper. Telephone 88 if supply defective Orbost Butter & Produce Co Ltd To Mrs C Tomlinson, 2 May 1952. Lighting15/- Power 5/3 Meter rent 1/- Account rendered 6/13/3orbost, electric power, orbost butter factory, c tomlinson -
 Ringwood and District Historical Society
Ringwood and District Historical SocietyPhotograph, Military Ball, Ringwood 1953
Typed below photograph, 'L. to R.: Sgt. W. Hall, Brig. H. H. Hamer (Brigade Commander) Major K. D. Green (O/C 10th Ind. Field Sqdn) - Photo by J. Gallagher'. 'Mail 18/12/53'. -
 Flagstaff Hill Maritime Museum and Village
Flagstaff Hill Maritime Museum and VillageDomestic object - Bowl, Late 19th or early 20th Century
The Process of Making Pottery Decorating, Firing, Glazing, Making, Technical There is a rhythm and flow to clay. It can’t be done all at once! Even the making process! It can take weeks to get everything done, especially if you can only work on your pottery once a week! Even though we have three hour classes, it’s often just not enough time! Here is an overview of some of the processes so you have a bit more grasp on some of the technical stuff! Step One – Design There are SO many ideas out there for making stuff in clay! From delicate porcelain jewellery, through to heavy sculptural work and everything in between. Deciding your direction is sometimes not that easy – when you first start, try everything, you will naturally gravitate to the style that you enjoy! The options and variations are endless and can get a wee bit overwhelming too! Check in with me before you start to ensure your ideas will work, what order you might do things, how you could achieve the look you are seeking and any other technical data required! Step Two – Making Clay is thixotropic. This means that as you work with it, the clay first gets sloppier and wetter, before is begins to dry in the atmosphere. For most things, you simply can’t do all parts of the project at once. An example of work order might look like: Get last weeks work out from the shelves Prepare clay for today’s work – roll your clay, prepare balls for throwing, make the first stage of a pinch pot) Clean up last week’s work and put it on the shelf for bisque firing Check that you have any glazing to do – and do enough of it that you will have time to finish your main project Do the next step of your next project – there might be a further step that can’t be complete immediately, in that case, wrap your work well and put onto the shelves. Letting your work rest for a while can really help keep your work clean and professional looking. Many things require bagging under plastic to keep it ready for work the next week – put your name on the outside of the bag so you can find your work easily. We have stickers and markers. Consider how you want to decorate your work – coloured slip can be applied at a fairly wet stage (remembering that it will make your work even wetter!). Trying to apply slip to dry clay won’t work! If you want to do sgraffito – you will need to keep the work leather hard (a state of dryness where you can still work the clay with a little effort and a little water and care). Step Three – Drying Most of the time your work can go into the rack uncovered to let it dry out for the following week. If you want to continue forming or shaping you will need to double bag your work – put your work on a suitable sized bat and put the bat in a bag so the base of the bag is under the bat, then put another bag over the top of the work and tuck the top of the bag under the bat. If you want to trim (or turn) your thrown work the following week, it should also be double bagged. If your work is large, delicate, or of uneven thicknesses, you should lightly cover your work for drying. When considering the drying process, bare in mind the weather, humidity and wind! The hotter and dryer, the faster things dry and work can dry unevenly in the shelves – this can lead to cracking – another time to lightly cover your work for drying. Step Four – Trimming and Cleaning Up Your work is dry! It is called greenware now and it is at it’s most fragile! Handle everything with two hands. I often refer to soft hands – keep everything gentle and with your fingers spread as much as possible. Try to not pick up things like plates too much, and always with both hands! Before your work can be bisque fired it should be “cleaned up”. You work won’t go into the kiln if it has sharp edges – when glazed, sharp edges turn into razor blades! Use a piece of fly wire to rub the work all over – this will scratch a little so be light handed. Use a knife or metal kidney to scrape any areas that require a bit more dynamic treatment than the fly wire offers! Finally, a very light wipe over with a slightly damp sponge can help soften and soothe all of your edges and dags! Trimming thrown work: If you are planning to trim (or turn) your thrown work (and you should be), make sure you bag it well – your work should be leather hard to almost dry for easiest trimming. Use this step to finish the work completely – use a metal kidney to polish the surface, or a slightly damp sponge to give a freshly thrown look. Wipe the sponge around the rim after trimming, and check the inside of the pot for dags! Trimming slip cast work: Usually I will trim the rims of your work on the wheel the following day to make that stage easier, however you will still need to check your work for lumps and bumps. Last but not least – check that your name is still clearly on the bottom of your work. Step Five – Bisque Firing When the work is completely dry it can go into the bisque kiln. The bisque kiln is fired to 1000°C. This process burns off the water in the clay as well as some of the chemically bound water. The structure of the clay is not altered that much at this temperature. Inside the bisque kiln, the work is stacked a little, small bowl inside a larger bowl and onto a heavy plate. Smaller items like decorations or drink coasters might get stacked several high. Consideration is paid to the weight of the stack and shape of the work. A bisque kiln can fire about one and a half times the amount of work that the glaze kiln can fire. The firing takes about 10 hours to complete the cycle and about two days to cool down. Once it has been emptied the work is placed in the glaze room ready for you to decorate! Step Six – Glazing Decorating your work with colour can be a lot of fun – and time consuming! There are three main options for surface treatment at this stage: Oxide Washes Underglazes Glazes Washes and underglazes do not “glaze” the work – It will still need a layer of glaze to fully seal the clay (washes don’t need glaze on surfaces not designed for food or liquid as they can gloss up a little on their own). Underglazes are stable colourants that turn out pretty much how they look in the jar. They can be mixed with each other to form other colours and can be used like water colours to paint onto your work. Mostly they should have a clear glaze on top to seal them. Oxides are a different species – the pink oxide (cobalt) wash turns out bright blue for instance. They don’t always need a glaze on top, and some glazes can change the colour of the wash! The glazes need no other “glaze” on top! Be careful of unknown glaze interactions – you can put any combination of glaze in a bowl or on a plate, but only a single glaze on the outside of any vertical surface! Glazes are a chemical reaction under heat. We don’t know the exact chemicals in the Mayco glazes we use. I can guess by the way they interact with each other, however, on the whole, you need to test every idea you have, and not run the test on a vertical surface! Simply put, glaze is a layer of glass like substance that bonds with the clay underneath. Clay is made of silica, alumina and water. Glaze is made of mostly silica. Silica has a melting point of 1700°C and we fire to 1240°C. The silica requires a “flux” to help it melt at the lower temperature. Fluxes can be all sorts of chemicals – a common one is calcium – calcium has a melting point of 2500°C, however, together they both melt at a much lower temperature! Colourants are metal oxides like cobalt (blue), chrome (green through black), copper (green, blue, even red!), manganese (black, purple and pink) iron (red brown), etc. Different chemicals in the glaze can have dramatic effects. for example, barium carbonate (which we don’t use) turns manganese bright pink! Other elements can turn manganese dioxide brown, blue, purple and reddish brown. Manganese dioxide is a flux in and of itself as well. So, glazes that get their black and purple colours, often interact with other glazes and RUN! Our mirror black is a good example – it mixes really well with many glazes because it fluxes them – causes them to melt faster. It will also bring out many beautiful colours in the glazes because it’s black colouring most definitely comes from manganese dioxide! Glaze chemistry is a whole subject on it’s own! We use commercial Mayco glazes on purpose – for their huge range of colour possibilities, stability, cool interactions, artistic freedom with the ability to easily brush the glazes on and ease of use. We currently have almost 50 glazes on hand! A major project is to test the interactions of all glazes with each other. That is 2,500 test tiles!!!! I’m going to make the wall behind the wheels the feature wall of pretty colours! Step Seven – Glaze (Gloss or sometimes called “Glost”) Firing Most of the time this is the final stage of making your creation (but not always!) The glaze kiln goes to 1240°C. This is called cone 6, or midrange. It is the low end of stoneware temperatures. Stoneware clays and glazes are typically fired at cone 8 – 10, that is 1260 – 1290°C. The energy requirement to go from 1240°C to 1280°C is almost a 30% more! Our clay is formulated to vitrify (mature, turn “glass-like”) at 1240°, as are our glazes. A glaze kiln take around 12 hours to reach temperature and two to three days to cool down. Sometimes a third firing process is required – this is for decoration that is added to work after the glaze firing. For example – adding precious metals and lustres. this firing temperature is usually around 600 – 800°C depending upon the techniques being used. There are many students interested in gold and silver trims – we will be doing this third type of firing soon! After firing your work will be in the student finished work shelves. Remember to pay for it before you head out the door! There is a small extra charge for using porcelain clay (it’s more than twice the price of regular clay), and for any third firing process! Once your work has been fired it can not turn back into clay for millennia – so don’t fire it if you don’t like it! Put it in the bucket for recycling. https://firebirdstudios.com.au/the-process-of-making-pottery/ The bowl is an example of kitchenware used in the 19th century and still in use today.Bowl white ceramic. Crack on side. Badly stained.Backstamp very faint and unable to be read.flagstaff hill, warrnambool, shipwrecked-coast, flagstaff-hill, flagstaff-hill-maritime-museum, maritime-museum, shipwreck-coast, flagstaff-hill-maritime-village, mixing bowl, food preparation, kitchen equipment, ceramic -
 Flagstaff Hill Maritime Museum and Village
Flagstaff Hill Maritime Museum and VillageDomestic object - Bowl
The Process of Making Pottery Decorating, Firing, Glazing, Making, Technical There is a rhythm and flow to clay. It can’t be done all at once! Even the making process! It can take weeks to get everything done, especially if you can only work on your pottery once a week! Even though we have three hour classes, it’s often just not enough time! Here is an overview of some of the processes so you have a bit more grasp on some of the technical stuff! Step One – Design There are SO many ideas out there for making stuff in clay! From delicate porcelain jewellery, through to heavy sculptural work and everything in between. Deciding your direction is sometimes not that easy – when you first start, try everything, you will naturally gravitate to the style that you enjoy! The options and variations are endless and can get a wee bit overwhelming too! Check in with me before you start to ensure your ideas will work, what order you might do things, how you could achieve the look you are seeking and any other technical data required! Step Two – Making Clay is thixotropic. This means that as you work with it, the clay first gets sloppier and wetter, before is begins to dry in the atmosphere. For most things, you simply can’t do all parts of the project at once. An example of work order might look like: Get last weeks work out from the shelves Prepare clay for today’s work – roll your clay, prepare balls for throwing, make the first stage of a pinch pot) Clean up last week’s work and put it on the shelf for bisque firing Check that you have any glazing to do – and do enough of it that you will have time to finish your main project Do the next step of your next project – there might be a further step that can’t be complete immediately, in that case, wrap your work well and put onto the shelves. Letting your work rest for a while can really help keep your work clean and professional looking. Many things require bagging under plastic to keep it ready for work the next week – put your name on the outside of the bag so you can find your work easily. We have stickers and markers. Consider how you want to decorate your work – coloured slip can be applied at a fairly wet stage (remembering that it will make your work even wetter!). Trying to apply slip to dry clay won’t work! If you want to do sgraffito – you will need to keep the work leather hard (a state of dryness where you can still work the clay with a little effort and a little water and care). Step Three – Drying Most of the time your work can go into the rack uncovered to let it dry out for the following week. If you want to continue forming or shaping you will need to double bag your work – put your work on a suitable sized bat and put the bat in a bag so the base of the bag is under the bat, then put another bag over the top of the work and tuck the top of the bag under the bat. If you want to trim (or turn) your thrown work the following week, it should also be double bagged. If your work is large, delicate, or of uneven thicknesses, you should lightly cover your work for drying. When considering the drying process, bare in mind the weather, humidity and wind! The hotter and dryer, the faster things dry and work can dry unevenly in the shelves – this can lead to cracking – another time to lightly cover your work for drying. Step Four – Trimming and Cleaning Up Your work is dry! It is called greenware now and it is at it’s most fragile! Handle everything with two hands. I often refer to soft hands – keep everything gentle and with your fingers spread as much as possible. Try to not pick up things like plates too much, and always with both hands! Before your work can be bisque fired it should be “cleaned up”. You work won’t go into the kiln if it has sharp edges – when glazed, sharp edges turn into razor blades! Use a piece of fly wire to rub the work all over – this will scratch a little so be light handed. Use a knife or metal kidney to scrape any areas that require a bit more dynamic treatment than the fly wire offers! Finally, a very light wipe over with a slightly damp sponge can help soften and soothe all of your edges and dags! Trimming thrown work: If you are planning to trim (or turn) your thrown work (and you should be), make sure you bag it well – your work should be leather hard to almost dry for easiest trimming. Use this step to finish the work completely – use a metal kidney to polish the surface, or a slightly damp sponge to give a freshly thrown look. Wipe the sponge around the rim after trimming, and check the inside of the pot for dags! Trimming slip cast work: Usually I will trim the rims of your work on the wheel the following day to make that stage easier, however you will still need to check your work for lumps and bumps. Last but not least – check that your name is still clearly on the bottom of your work. Step Five – Bisque Firing When the work is completely dry it can go into the bisque kiln. The bisque kiln is fired to 1000°C. This process burns off the water in the clay as well as some of the chemically bound water. The structure of the clay is not altered that much at this temperature. Inside the bisque kiln, the work is stacked a little, small bowl inside a larger bowl and onto a heavy plate. Smaller items like decorations or drink coasters might get stacked several high. Consideration is paid to the weight of the stack and shape of the work. A bisque kiln can fire about one and a half times the amount of work that the glaze kiln can fire. The firing takes about 10 hours to complete the cycle and about two days to cool down. Once it has been emptied the work is placed in the glaze room ready for you to decorate! Step Six – Glazing Decorating your work with colour can be a lot of fun – and time consuming! There are three main options for surface treatment at this stage: Oxide Washes Underglazes Glazes Washes and underglazes do not “glaze” the work – It will still need a layer of glaze to fully seal the clay (washes don’t need glaze on surfaces not designed for food or liquid as they can gloss up a little on their own). Underglazes are stable colourants that turn out pretty much how they look in the jar. They can be mixed with each other to form other colours and can be used like water colours to paint onto your work. Mostly they should have a clear glaze on top to seal them. Oxides are a different species – the pink oxide (cobalt) wash turns out bright blue for instance. They don’t always need a glaze on top, and some glazes can change the colour of the wash! The glazes need no other “glaze” on top! Be careful of unknown glaze interactions – you can put any combination of glaze in a bowl or on a plate, but only a single glaze on the outside of any vertical surface! Glazes are a chemical reaction under heat. We don’t know the exact chemicals in the Mayco glazes we use. I can guess by the way they interact with each other, however, on the whole, you need to test every idea you have, and not run the test on a vertical surface! Simply put, glaze is a layer of glass like substance that bonds with the clay underneath. Clay is made of silica, alumina and water. Glaze is made of mostly silica. Silica has a melting point of 1700°C and we fire to 1240°C. The silica requires a “flux” to help it melt at the lower temperature. Fluxes can be all sorts of chemicals – a common one is calcium – calcium has a melting point of 2500°C, however, together they both melt at a much lower temperature! Colourants are metal oxides like cobalt (blue), chrome (green through black), copper (green, blue, even red!), manganese (black, purple and pink) iron (red brown), etc. Different chemicals in the glaze can have dramatic effects. for example, barium carbonate (which we don’t use) turns manganese bright pink! Other elements can turn manganese dioxide brown, blue, purple and reddish brown. Manganese dioxide is a flux in and of itself as well. So, glazes that get their black and purple colours, often interact with other glazes and RUN! Our mirror black is a good example – it mixes really well with many glazes because it fluxes them – causes them to melt faster. It will also bring out many beautiful colours in the glazes because it’s black colouring most definitely comes from manganese dioxide! Glaze chemistry is a whole subject on it’s own! We use commercial Mayco glazes on purpose – for their huge range of colour possibilities, stability, cool interactions, artistic freedom with the ability to easily brush the glazes on and ease of use. We currently have almost 50 glazes on hand! A major project is to test the interactions of all glazes with each other. That is 2,500 test tiles!!!! I’m going to make the wall behind the wheels the feature wall of pretty colours! Step Seven – Glaze (Gloss or sometimes called “Glost”) Firing Most of the time this is the final stage of making your creation (but not always!) The glaze kiln goes to 1240°C. This is called cone 6, or midrange. It is the low end of stoneware temperatures. Stoneware clays and glazes are typically fired at cone 8 – 10, that is 1260 – 1290°C. The energy requirement to go from 1240°C to 1280°C is almost a 30% more! Our clay is formulated to vitrify (mature, turn “glass-like”) at 1240°, as are our glazes. A glaze kiln take around 12 hours to reach temperature and two to three days to cool down. Sometimes a third firing process is required – this is for decoration that is added to work after the glaze firing. For example – adding precious metals and lustres. this firing temperature is usually around 600 – 800°C depending upon the techniques being used. There are many students interested in gold and silver trims – we will be doing this third type of firing soon! After firing your work will be in the student finished work shelves. Remember to pay for it before you head out the door! There is a small extra charge for using porcelain clay (it’s more than twice the price of regular clay), and for any third firing process! Once your work has been fired it can not turn back into clay for millennia – so don’t fire it if you don’t like it! Put it in the bucket for recycling. https://firebirdstudios.com.au/the-process-of-making-pottery/ This bowl is an example of kitchenware used in the 19th century and still in use today.Bowl white ceramic plain that has two sets of edging around lip. Inside bowl has plaster designed to look like cooking mixture.flagstaff hill, warrnambool, shipwrecked-coast, flagstaff-hill, flagstaff-hill-maritime-museum, maritime-museum, shipwreck-coast, flagstaff-hill-maritime-village, kitchen equipment, ceramic -
 Flagstaff Hill Maritime Museum and Village
Flagstaff Hill Maritime Museum and VillageDomestic object - Bowl, J & G Meakin, Late 19th or early 20th Century
The Process of Making Pottery Decorating, Firing, Glazing, Making, Technical There is a rhythm and flow to clay. It can’t be done all at once! Even the making process! It can take weeks to get everything done, especially if you can only work on your pottery once a week! Even though we have three hour classes, it’s often just not enough time! Here is an overview of some of the processes so you have a bit more grasp on some of the technical stuff! Step One – Design There are SO many ideas out there for making stuff in clay! From delicate porcelain jewellery, through to heavy sculptural work and everything in between. Deciding your direction is sometimes not that easy – when you first start, try everything, you will naturally gravitate to the style that you enjoy! The options and variations are endless and can get a wee bit overwhelming too! Check in with me before you start to ensure your ideas will work, what order you might do things, how you could achieve the look you are seeking and any other technical data required! Step Two – Making Clay is thixotropic. This means that as you work with it, the clay first gets sloppier and wetter, before is begins to dry in the atmosphere. For most things, you simply can’t do all parts of the project at once. An example of work order might look like: Get last weeks work out from the shelves Prepare clay for today’s work – roll your clay, prepare balls for throwing, make the first stage of a pinch pot) Clean up last week’s work and put it on the shelf for bisque firing Check that you have any glazing to do – and do enough of it that you will have time to finish your main project Do the next step of your next project – there might be a further step that can’t be complete immediately, in that case, wrap your work well and put onto the shelves. Letting your work rest for a while can really help keep your work clean and professional looking. Many things require bagging under plastic to keep it ready for work the next week – put your name on the outside of the bag so you can find your work easily. We have stickers and markers. Consider how you want to decorate your work – coloured slip can be applied at a fairly wet stage (remembering that it will make your work even wetter!). Trying to apply slip to dry clay won’t work! If you want to do sgraffito – you will need to keep the work leather hard (a state of dryness where you can still work the clay with a little effort and a little water and care). Step Three – Drying Most of the time your work can go into the rack uncovered to let it dry out for the following week. If you want to continue forming or shaping you will need to double bag your work – put your work on a suitable sized bat and put the bat in a bag so the base of the bag is under the bat, then put another bag over the top of the work and tuck the top of the bag under the bat. If you want to trim (or turn) your thrown work the following week, it should also be double bagged. If your work is large, delicate, or of uneven thicknesses, you should lightly cover your work for drying. When considering the drying process, bare in mind the weather, humidity and wind! The hotter and dryer, the faster things dry and work can dry unevenly in the shelves – this can lead to cracking – another time to lightly cover your work for drying. Step Four – Trimming and Cleaning Up Your work is dry! It is called greenware now and it is at it’s most fragile! Handle everything with two hands. I often refer to soft hands – keep everything gentle and with your fingers spread as much as possible. Try to not pick up things like plates too much, and always with both hands! Before your work can be bisque fired it should be “cleaned up”. You work won’t go into the kiln if it has sharp edges – when glazed, sharp edges turn into razor blades! Use a piece of fly wire to rub the work all over – this will scratch a little so be light handed. Use a knife or metal kidney to scrape any areas that require a bit more dynamic treatment than the fly wire offers! Finally, a very light wipe over with a slightly damp sponge can help soften and soothe all of your edges and dags! Trimming thrown work: If you are planning to trim (or turn) your thrown work (and you should be), make sure you bag it well – your work should be leather hard to almost dry for easiest trimming. Use this step to finish the work completely – use a metal kidney to polish the surface, or a slightly damp sponge to give a freshly thrown look. Wipe the sponge around the rim after trimming, and check the inside of the pot for dags! Trimming slip cast work: Usually I will trim the rims of your work on the wheel the following day to make that stage easier, however you will still need to check your work for lumps and bumps. Last but not least – check that your name is still clearly on the bottom of your work. Step Five – Bisque Firing When the work is completely dry it can go into the bisque kiln. The bisque kiln is fired to 1000°C. This process burns off the water in the clay as well as some of the chemically bound water. The structure of the clay is not altered that much at this temperature. Inside the bisque kiln, the work is stacked a little, small bowl inside a larger bowl and onto a heavy plate. Smaller items like decorations or drink coasters might get stacked several high. Consideration is paid to the weight of the stack and shape of the work. A bisque kiln can fire about one and a half times the amount of work that the glaze kiln can fire. The firing takes about 10 hours to complete the cycle and about two days to cool down. Once it has been emptied the work is placed in the glaze room ready for you to decorate! Step Six – Glazing Decorating your work with colour can be a lot of fun – and time consuming! There are three main options for surface treatment at this stage: Oxide Washes Underglazes Glazes Washes and underglazes do not “glaze” the work – It will still need a layer of glaze to fully seal the clay (washes don’t need glaze on surfaces not designed for food or liquid as they can gloss up a little on their own). Underglazes are stable colourants that turn out pretty much how they look in the jar. They can be mixed with each other to form other colours and can be used like water colours to paint onto your work. Mostly they should have a clear glaze on top to seal them. Oxides are a different species – the pink oxide (cobalt) wash turns out bright blue for instance. They don’t always need a glaze on top, and some glazes can change the colour of the wash! The glazes need no other “glaze” on top! Be careful of unknown glaze interactions – you can put any combination of glaze in a bowl or on a plate, but only a single glaze on the outside of any vertical surface! Glazes are a chemical reaction under heat. We don’t know the exact chemicals in the Mayco glazes we use. I can guess by the way they interact with each other, however, on the whole, you need to test every idea you have, and not run the test on a vertical surface! Simply put, glaze is a layer of glass like substance that bonds with the clay underneath. Clay is made of silica, alumina and water. Glaze is made of mostly silica. Silica has a melting point of 1700°C and we fire to 1240°C. The silica requires a “flux” to help it melt at the lower temperature. Fluxes can be all sorts of chemicals – a common one is calcium – calcium has a melting point of 2500°C, however, together they both melt at a much lower temperature! Colourants are metal oxides like cobalt (blue), chrome (green through black), copper (green, blue, even red!), manganese (black, purple and pink) iron (red brown), etc. Different chemicals in the glaze can have dramatic effects. for example, barium carbonate (which we don’t use) turns manganese bright pink! Other elements can turn manganese dioxide brown, blue, purple and reddish brown. Manganese dioxide is a flux in and of itself as well. So, glazes that get their black and purple colours, often interact with other glazes and RUN! Our mirror black is a good example – it mixes really well with many glazes because it fluxes them – causes them to melt faster. It will also bring out many beautiful colours in the glazes because it’s black colouring most definitely comes from manganese dioxide! Glaze chemistry is a whole subject on it’s own! We use commercial Mayco glazes on purpose – for their huge range of colour possibilities, stability, cool interactions, artistic freedom with the ability to easily brush the glazes on and ease of use. We currently have almost 50 glazes on hand! A major project is to test the interactions of all glazes with each other. That is 2,500 test tiles!!!! I’m going to make the wall behind the wheels the feature wall of pretty colours! Step Seven – Glaze (Gloss or sometimes called “Glost”) Firing Most of the time this is the final stage of making your creation (but not always!) The glaze kiln goes to 1240°C. This is called cone 6, or midrange. It is the low end of stoneware temperatures. Stoneware clays and glazes are typically fired at cone 8 – 10, that is 1260 – 1290°C. The energy requirement to go from 1240°C to 1280°C is almost a 30% more! Our clay is formulated to vitrify (mature, turn “glass-like”) at 1240°, as are our glazes. A glaze kiln take around 12 hours to reach temperature and two to three days to cool down. Sometimes a third firing process is required – this is for decoration that is added to work after the glaze firing. For example – adding precious metals and lustres. this firing temperature is usually around 600 – 800°C depending upon the techniques being used. There are many students interested in gold and silver trims – we will be doing this third type of firing soon! After firing your work will be in the student finished work shelves. Remember to pay for it before you head out the door! There is a small extra charge for using porcelain clay (it’s more than twice the price of regular clay), and for any third firing process! Once your work has been fired it can not turn back into clay for millennia – so don’t fire it if you don’t like it! Put it in the bucket for recycling. https://firebirdstudios.com.au/the-process-of-making-pottery/This bowl was made by renowned pottery company J & G Meakin of England. The firm was established in the mid-1800's. The bowl is an example of kitchenware used in the 19th century and still in use today.Bowl; white ceramic, round and tapering inwards towards base. Made by J and G Meakin England.On base, 'Ironstone China Reg SOL 391413' with symbolflagstaff hill, flagstaff hill maritime museum and village, warrnambool, maritime museum, maritime village, great ocean road, shipwreck coast, mixing bowl, food preparation, j & g meakin, pottery, stoke-on-trent, kitchen equipment, ceramic -
 Glen Eira Historical Society
Glen Eira Historical SocietyDocument - SHELFORD GIRLS’ SCHOOL AND KINDERGARTEN
This file contains 2 items relating to the History of Shelford Girls’ School and Kindergarten: 1/1 set of typed notes by Betty Snowball, titled ‘History of Shelford Church of England Girls’ Grammar School’, date unknown. The notes detail the history of the school from 1898 to 1973 including a period from 1922 when boys and girls were enrolled. 2/2 copies of typed notes detailing the history of the school from 1898 to 1982. Author and date unknown.dixon emily miss, shelford church of england girls’ grammar school, ‘shelford’, glen eira road, st mary’s church, seymour road, blundell d miss, homain freda miss, schools, blundell f miss, alison road, langley h.t. canon, green bishop, anglican church, thomas a.m. miss, principals, students, bishops, ‘helenslea’, hood crescent, hood judge, ripley grove, st margaret’s hall, thewlis d miss, foundation stones, criswick wing, criswick mrs, cooper wing, cooper canon, woods f archbishop dr, baddams v.t. miss, baddams wing, cowling c.c. reverend, washington i.i. miss, ida washington library, delacombe rohan sir major general (governor), myers j.m. miss, parents clubs, classrooms, schoolhouses, davies margaret e miss, nicholson mabel miss, the nicholson primary library, st mary’s parish caulfield, caulfield, lloyd c mr, thewlis wing, henderson g mr, henderson mrs, hancock archdeacon, gates, watson normie, scholl reginald sir, gorton john senator, langley canon, langley dean, hone brian sir, caulfield grammar, excursions, construction (events and activities), britten e. miss, halls, theatres, ‘little shelford’, thewlis j.s. miss, coach houses, lofts, festivals and celebrations, primary schools, secondary schools, stables, clergy residences, fireplaces, towers, mansions -
 Glen Eira Historical Society
Glen Eira Historical SocietyLetter - Kirkham, C. F
... . Councillors Majors Caulfield Jowett F. Town Clerk Lloyd C. Mayor ...This file contains four items. 1. An original handwritten letter to Councillor Kirkham, congratulating him on his valuable services as Mayor of Caulfield for the year 1905, dated 31/08/1906. 2. Research by Claire Barton, on 22/02/2013 from Sands and McDougall’s directories showing that C. F. Kirkham lived from 1903 to 1923 in Gladstone Parade, Elsternwick in a house named ‘Hartington’. 3. Leather bound address book with C. Kirkham Elsternwick, 22/10/1999, name written inside with one short diary entry under ‘V’ only. 4. Admittance ticket to the Coronation of their Majesties King George V and Queen Mary, 22/06/2911, for C. F. Kirkham.kirkham c. f., councillors, majors, caulfield, jowett f. town clerk, lloyd c. mayor, gladstone parade, elsternwick, ‘hartington’, house names, kirkham charles f., diaries, journals, coronation celebrations, invitations -
 Glen Eira Historical Society
Glen Eira Historical SocietyNewsletter - CAULFIELD RSL
This file contains one item about the Caulfield RSL’s newsletter: 1/Five issues (nos. 26, 33, 35, 42, and 44) of Furfs, the official newsletter of the Caulfield RSL, dated 12/2000, 05/2004, 10/2005, 12/2007, and 11/2009 respectively. The issues vary in size (numbering 12, 6, 1, 8, and 8 pages, respectively) and consequently, in terms of breadth of content. Most, however, report on the Club’s recent activities, list the current administrative staff, eulogise recently deceased members, and contain advertisements for goods and services of interest to members. All except the shortest also contain numerous black-and-white photographs of members participating in various events. No. 42 also has an article on the progress of the Club history including mention of a 1963 ABC Four Corners episode with footage inside and outside the Club as well as interviews with members.furfs, advertisements, gould tony, moore hedley, frances ken, stewart r. k, whybrow john, mclean tom, elsternwick, canfield bill, anzac day, remembrance day, middlemiss glenys, middlemiss brian, sayers john, rodda alby, st. george’s road, remembrance day service, president’s dinner, annett michael colonel, dejussing clive, stacey carole, white thomas sir, fuller joan, edwards m. t., greer j., warburton jimmy, decker john, mcbean j., mclean s. mrs., gibson ray, rigby jack, oakley trevor, paul’s steak house, wear well dry cleaners, mclean susan, caulfield lions clubs, glen eira district lions, ‘my brother jack’ short story award, ‘glenmore’, everett kevin, lee kenneth c., sneddon bruce n., perignon george j., bradley john m., kennedy colin j., waratah cellars, glenhuntly road, glen huntly road, taverna john, taverna robyn, gaylard bernie, auhl ron, yob loretta, muir frank, morris tony, blaney matt, james bob, kevin brennan, davey kathleen, wreath laying ceremonies, may carl, one petroleum co., ager michelle major, taus joe, geier noel, blore peter, creaney john, wadley geoffrey, oliver mavis i., browne donald d., ross john, kidd william, rea alan m., pollock rob, pollock florence, sell patricia miss, fidler n., dew d., mayell w., booth vincent, booth lois, johnson carl, ‘the ole tin hat’, centre road, bentleigh, rigby pat, eade charles, howell davie, blore jeff, russell paul, kesoglidis kon, morse r. e., long bridford f. r., harkensee keith r., collins williams h., daniels albert e., stephani detleef p. von, walton alan l., canfield william g., barclay henry i., rose frank w., sherriff william g., may donald c., fergus mark l., sorel peter a., chivers keith, scarffe richard a., dalgleish maggie, sampson stan, jacobs frank, ware les, powell massey, merlo harry, perini leo, milnes ‘bluey’, carmody jack, edwards michael terrence, orrock george, lyon stan, raines barbara, raines laurie, hall ron, metherall murray, blore geoff, walker keith, davis max, doyle tom, reece jim, jewell bill, o’neill roe, ferrari ron, fogarty des, sinclair bill, mitchell j., harris l., boughton h. ms., townsend d. mrs., decker j., larkin r., elder g., sell p., clarke john, mclean t., kean phyllis m., booth geoffrey e., donoghue clifton s., durham leonard c., ford john w., nitsche neil h., davidson kelvin r., werba adele, pleydell max, price colin, taberner laurie, condron neil, leech gail, green barry rev’d, veteran’s lunch, devlin joseph h., murray mark blodwen, jackman david d., hall erica d., hackman james f., thompson pauline l., gassick betty m. le, logan george, wilson tony, slater bob, cobby a. h. (harry) air commodore, steinberg alison mrs., astill bob, reed donald, niewland hans, bodelier berry, little harold, hawkes stewart, limbue ram bahadur, kirkwood robert, rai bharansher, mclean susan, mcbean jon, white michael sgt., caspar freddie -
 Federation University Historical Collection
Federation University Historical CollectionBook, Extra Muros 1956, Teachers' College Ballarat, 1956
Editorial, Our Principal, Second Year Staff and Students', First Year Students', Main Events of '56, Hostel Highlights, Sporting World, Sports Awards. December 1955 Ballarat Teachers’ College held its first Graduation Ceremony. The words of the Graduation Hymn were written by Mavis Canty. (BTC Handbook 1965) July 1956 Tenders were called for the first section of the new Ballarat Teachers’ College to be built in Gillies St. (BTC Handbook 1965)Light blue cover (cream inside cover) with dark blue and yellow titles. Soft covered magazine of the Ballarat Teachers College. Title page states Vol. 2. No. 3.btc, ballarat teachers college, n. l. harvey, t. w. h. turner, john pianta, d. stanley, b. parker, j. hutchings, n. ruddick, b. poole, r. arthur, e. w. doney, f. golding, a. hutchings, j. hammet, d. wyley, m. hutchinson, i. burt, y. davey, p. mcconville, m. ryan, h. miller, p. wiltshire, d. wright, m. hyndman, v. goodwin, k. reither, h. weeks, r. delbridge, n. freeman, b. pointer, w. taylor, g. mcrae, l. dugdale, b. robson, e. o. walpole, r. j. croft, e. constable, j. brown, j. dugdale, ian burt, heather sparkes, g. whitelaw, e. major, ruth ray, ron bunn, w. j. taylor, shirley a. mclay, d. henderson, a. gleeson, p. utber, a. rutter, i. erdmanis, e. phillips, i. mckinley, a. stalker, m. spencer, c. rodger, r. ross, l. jenkins, j. m. blair, j. kirk, m. odd, b. dahlenburg, m. cattanach -
 Federation University Historical Collection
Federation University Historical CollectionMagazine - Booklet, Ballarat School of Mines Students' Magazine, 1952, 1952
School Council, Members of Staff, Editorial, Principal's Page, Magazine Committee, Obituary - Rupert P. Flower, Literary Society, Prominent Personalities, Science School, News and Notes, Prize Presentation, The Apprentices, Boys Sport, Sun Youth Travel Memoirs, The Art School, His Majesty the Late King George VI, Commercial Notes, Gleanings Here and There, Junior School, Girls Sport, Sport, House Notes, Junior Technical School Students', Roll Call - Diploma Students 1952Pale blue soft covered magazine with navy blue titles.ballarat school of mines, magazine, mr bryan, c. sanos, s. deans, j. williams, r. simpson, c. g. fairbank, d. treadwell, e. aitkens, g. birkett, g. allen, f. benjamin, s. gillespie, r. hullick, h. mccallum, h. harris, j. walton, e. walsh, j. stevens, b. clark, rupert p. flower, john bechervaise, w. keith hindson, a. james tinney, walter c. tooth, john d. bethune, vilma sansom, betty clark, travers duncan, joyce wilson, lex lockhart, jim w. beattie, joyce stevens, slim ingleton, a. eddy, john howard, douglas george dean, edgar bartrop, colin mck. henry, tom adams, max kennedy, jeff coward, o. j. nilsen, j. skuja, s. rowe, w. maddox, a. kinnane, d. fraser, a. carpenter, john james, b. flavel, j. murray, d. schmidt, g. habel, t. duncan, l. matthews, n. spiers, b. smith, a. tonnisseu, r. ingleton, j. bethune, j. mills, j. mcneil, b. schreenan, w. carlyon, d. stevens, l. j. hillman, k. hindson, r. furlong, j. beattie, b. taylor, g. heyes, l. quilliam, r. archer, a. johnson, m. gillin, t. seabrook, m. phillips, j. sawyer, c. restarick, j. saggers, g. ditchfield, j. tinney, don stewart, j. faneco, m. stevens, w. tooth, ron simpson, bill maxwell, graham searle, jim tinney, k. treloar, j. barnes, s. j. deans, lynette j. blomeley, georgina cox, heather mcgregor, janet saunders, heather harris, elizabeth mcarthur, norma coffield, pat lavery, imelda lee, gloria white, valerie westbrook, cynthia stone, janice thompson, clare mooney, glenys perry, faida lewis, betty clarke, marion volk, isla veal, valerie yates, coralie mckenzie, lorraine digby, barbara henderson, deidre wilson, margaret henderson, barbara ngip, glenys sleeth, anne duncan, l. dwyer, elaine leishman, j. jenkin, stirling gillespie, w. bridges, b. baldock, b. braybrook, e. mackie, r. braybrook, c. grose, b. mackie, c. garnham, c. schmidtke, t. lugg, j. copeman, b. tozer, g. mathews, n. sutherland, r. lambert, j. sanders, m. quick, j. collier, g. pike, r. digby, r. quayle, r. sharp, n. brogden, r. lyons, p. stevens, j. bastin, b. kay, k. duncan, b. golding, l. norman, b. murnane, k. hocking, c. sealey, b. langdon, f. weightman, m. birch, r. stevenson, r. stewart, r. haintz, k. mccoll, k. jarvis, l. hocking, d. curtain, r. lazarus, e. boak, j. fletcher, b. orchard, j. squires, n. pike, j. shrader, l. reynolds, m. ritchie, g. smith, d. parkes, g. templeton, m. wunhym, v. vincent, d. robertson, d. lang, l. horwood, d. searle, d. new, v. jolly, a. minotti, b. beaumont, m. marshall, e. bowen, j. rogers, d. cody, e. kinnane, j. cunningham, j. schrader, r. horgan, j. white, n. flood, j. matthews, h. gale, k. mitchell, v. rowse, j. mayne, a. gilbert, b. warrillow, g. gilbert, n. quick, m. hall, d. furlong, n. lyons, j. richards, j. jones, l. major, d. baldock, d. dow, g. ruddick, d. howell, j. caddy, b. singleton, b. powell, r. sharpe, c. lockhart, l. daff, c. sharpe, d. irish, l. dow, a. douglas, n. twaites, j. courtney, l. beacham, n. c. cartledege, cliff sealey, j. f. collier, e. g. mackie, m. g. quick, b. l. collinson, j. n. bastin, g. e. timmins, valerie mills, j. a. jenkin, w. cowan, i. mitaxa, n. c. leckie, r. j. austin, n. coffield, d. quilliam, r. courtney, f. m. kilfoyle, p. nunn, king george vi -
 Federation University Historical Collection
Federation University Historical CollectionPhotograph, Pioneers of Ballarat
The Pioneers of Ballarat may have been created to the Ballarat Old Colonists' Association and the reunions held by the early Ballarat pioneers. The dates given after the pioneers time is their date of arrival into the colony.Photograph showing numerous portraits of men who were considered Pioneers of Ballarat as complied by R. Walker and produced by Chuck Vice Regal Photo. The men depicted are: J. Smith; W. Gay; W. Downie; G. Goddard; B. Varcoe; A.F. Seidal; Loius Balhausen; J. McKenzie; William irwin; M. Box; Thomas Bath; James Oddie; William Tulloch; J.W. Graham; J. Ward; W. Curnow; R.J. Binder; F.J.L. Turner; W. Eyres; C.A. Welch; B. Welch; G. Welch; J. Lake; H. Smith; J. King; R.T. Wreford; Ernest Balhausen; J. Ward; T. Trengrove; J. Truswell; J. Taylor; W. Seeley; J.P. Murray; Hon. David Ham; Andrew Anderson; E. McCartney; J.H. Smith; Joseph Edward Cowley; A. Jones; W.H. Furness; F.N. Martin; James Anderson; G. Becher; James Meek; T. Hawkins; P. Drummond; C. Barker; J. Malcolm; R. Brown; G. Tupper; A. Rose; W. Pinkerton; A. Clinton; A. Sheppard, F.C. Downes; J.A. Blight; J. Blight; T. Blight; J. Richardson; C.W. Toy; W.C. Elder; E. Towl; S. Walker; W.P. Martin; J.T Langley; J.A. Abramowitch; R, Allan; S.W. Woodward; G. Hunt; J. Bishop; F.L. Graham; R. Graham; W. A.G. Fraser; J. Davies; J. Paterson; B.G. Tucker; E. McElroy; W.H. Burton, T.C. Coates; J. Williams; J.P. Roberts; J. Ritchie; T.W. White; J. F. Harvey; Natale D'Angri; D. McCallum; W. Chisholm; P. Kohl; J. Moncrief; J.P. Marshall; R.U. Nicholls; G.L. Holthouse; C. Gray; W. Gale; James Long; Theo Williams; J.R. Ellsworth; W. Scott; Henry Josephs; D. Cooke; William Little; T.H. Thompson; E. Morey; J.G. McDonald; C.C. Shoppee; G. Douglas; W. H. Ellis; W. Hicks; J. Cameron; W. B. Koppers; F. Marendez; G. Herrmann; J. Kelly; E. Jermyn; P. Murray; P. Gay; D. McNaught; T.D. Wanliss; G. Lord; H. Glenny; J. Trethowan; J. Blandford; J. Goujon; W. Coad; A. Colliver; J. Nunn; J. Munro; W.C. Burbidge; J. Jarvie; C. Ferguson; C. Morris; J. Russell; J. Phillips; J. Coghlan; R. Clark; Thomas Stoddart; M. Wasley; B. Retallack; John Reid; M.C. Carey; P. Maloney; E. Newman; J. Lamb; J,. Pryor; J. Gibson; James Mitchell; J. Rowe; James Vallins; A. Roxburgh; A. Cant; O. Thomas; J.Y. McDonald; W.M. Acheson; A. Jack; R. Gibbings; E.W. Chamberlain; J.H. Ellsworth; J. Falconer; G.G. Lorimer; James M. Bickett; T. Sayle; Andrew McIntyre; W. Hambley; K. Coutts; T. Muir; R. Scott; G. Leach; E. Richards; R. Hearn; J. Hughan; D. Miliani; E. Parr; J. T. Irving; W.G. Williams; J. Marks; J. Darby; T. Ray; D. McKenzie; James Robson; J. Robson; J. Moore; J. Murphy; Robert M. Serjeant; C. Ford; E.E. Campbell; P. Folland; P.J. Rickard; A. McVitty; B. Angwin; J.T. Sleep; M.P. Whiteside; W. Curtis; H. Crisp; E. Major; R. Pearce; J. Waller; G. Waller; G. Abrams; J. McIntyre; J. Johnston; W. Johnston; W. Taylor; J. Knoth; J. Davey; G. Smith; N. Kent; E.O. Witherden; J.B. Cathcart; W.H. Harrow; G. Evans; L. Ure; W.T. Glen; T. Dickinson; D. Hughes; J. Strickland; J. Hillman; E. Jackson; R.J. Walker; D. Gunn; R.J. Gullan; T. McManamy; A. Gray; James trembath; W. Porter; J. Showman; C. Walker; J. Bowman; W.B. McDonald; P. Jago; J. Stout pioneers, ballarat, chuck, chuck vice regal photo, r. walker, ballarat pioneers, pioneers of ballarat, j. smith, w. gay, w. downie, g. goddard, b. varcoe, a.f. seidal, loius balhausen, j. mckenzie, william irwin, m. box, thomas bath, james oddie, william tulloch, j.w. graham, j. ward, w. curnow, r.j. binder, f.j.l. turner, w. eyres, c.a. welch, b. welch, g. welch, j. lake, h. smith, j. king, r.t. wreford, ernest balhausen, t. trengrove, j. truswell, j. taylor, w. seeley, j.p. murray, hon. david ham, andrew anderson, e. mccartney, j.h. smith, joseph edward cowley, a. jones, w.h. furness, f.n. martin, james anderson, g. becher, james meek, t. hawkins, p. drummond, c. barker, j. malcolm, r. brown, g. tupper, a. rose, w. pinkerton, a. clinton, a. sheppard, f.c. downes, j.a. blight, j. blight, t. blight, j. richardson, c.w. toy, w.c. elder, e. towl, s. walker, w.p. martin, j.t langley, j.a. abramowitch, r, allan, s.w. woodward, g. hunt, j. bishop, f.l. graham, r. graham, w. a.g. fraser, j. davies, j. paterson, b.g. tucker, e. mcelroy, w.h. burton, t.c. coates, j. williams, j.p. roberts, j. ritchie, t.w. white, j. f. harvey, natale d'angri, d. mccallum, w. chisholm, p. kohl, j. moncrief, j.p. marshall, r.u. nicholls, g.l. holthouse, c. gray, w. gale, james long, theo williams, j.r. ellsworth, w. scott, henry josephs, d. cooke, william little, t.h. thompson, e. morey, j.g. mcdonald, c.c. shoppee, g. douglas, w. h. ellis, w. hicks, j. cameron, w. b. koppers, f. marendez, g. herrmann, j. kelly, e. jermyn, p. murray, p. gay, d. mcnaught, t.d. wanliss, g. lord, h. glenny, j. trethowan, j. blandford, j. goujon, w. coad, a. colliver, j. nunn, j. munro, w.c. burbidge, j. jarvie, c. ferguson, c. morris, j. russell, j. phillips, j. coghlan, r. clark, thomas stoddart, m. wasley, b. retallack, john reid, m.c. carey, p. maloney, e. newman, j. lamb, j, . pryor, j. gibson, james mitchell, j. rowe, james vallins, a. roxburgh, a. cant, o. thomas, j.y. mcdonald, w.m. acheson, a. jack, r. gibbings, e.w. chamberlain, j.h. ellsworth, j. falconer, g.g. lorimer, james m. bickett, t. sayle, andrew mcintyre, w. hambley, k. coutts, t. muir, r. scott, g. leach, e. richards, r. hearn, j. hughan, d. miliani, e. parr, j. t. irving, w.g. williams, j. marks, j. darby, t. ray, d. mckenzie, james robson, j. robson, j. moore, j. murphy, robert m. serjeant, c. ford, e.e. campbell, p. folland, p.j. rickard, a. mcvitty, b. angwin, j.t. sleep, m.p. whiteside, w. curtis, h. crisp, e. major, r. pearce, j. waller, g. waller, g. abrams, j. mcintyre, j. johnston, w. johnston, w. taylor, j. knoth, j. davey, g. smith, n. kent, e.o. witherden, j.b. cathcart, w.h. harrow, g. evans, l. ure, w.t. glen, t. dickinson, d. hughes, j. strickland, j. hillman, e. jackson, r.j. walker, d. gunn, r.j. gullan, t. mcmanamy, a. gray, james trembath, w. porter, j. showman, c. walker, j. bowman, w.b. mcdonald, p. jago, j. stout, john smith -
 Federation University Historical Collection
Federation University Historical CollectionPostcard - Sepia, Miss Tittell Brune, c1905, c1905
Minnie Tittell Brune was born in 1875 and died in 1974. She was an American actress, little known in her own country, but a major figure in the history of the Australian stage. The peak of her career coincided with an Antipodean tour from 1904 to 1909. She was brought to Australia by J. C. Williamson, A sepia postard whowing a seatedwoman in elaborate costume. She is American actress Minnie Tittle Brunechatham-holmes family collection, tittell brune, actress, theatre -
 Federation University Historical Collection
Federation University Historical CollectionMagazine, Ballarat School of Mines Students' Magazine, 1898-1901, 1898-1901
Bound copies of the Ballarat School of Mines Students' Magazine, 1898-1901 Vol 1, No. 1, September 1898 * News and Notes (Ballarat School of Mines Museum, J.F. Usher, New British Pharmacopoeia, excursion to Bendigo) * History of the Ballarat School of Mines * Current Topics (Federation, Gladstone, Anglo-American Alliance) * Of Custom * Discovery of Coolgardie * Mining Notes(Clunes, Pitfield, Birthday Mine, Western Australia, Transvaal, Mt Bischoff, Rand Drill Co.) * From the Journals * The Societies - (Student Association, Ballarat Field Club and Science Society, Ballarat Photographic Club) * Original Poetry * Sports * Students' Association Committee Meetings * On the Increase of Temperature of the Earth With Increased Depth Vol 1, No. 2, October 1898 * Notes about some of the Past Students (E.M. Weston, J.A. Porter, H.R. Sleeman, G.E. Sander, B.C.T. Solley, T. Rhys, C. Burbury, D. McDougal, J. Matsen) * Excursion to Daylesford, p.3 * History of the Ballarat School of Mines (continued) * The Soudan * Greater Melbourne * Image of J. Hopkinson, electrical engineer killed ascending the Alps * What is Science * Mining Notes (Pitfield Plains, Victoria United G.M.Co., Lithgow, Avoca, great Cobar, Mt Whycheproof) * Student's Association (women's franchise) * Sports Vol 2, No. 1, March 1899 * News and Notes * History of the Ballarat School of Mines (continued) * Notes of Victorian Geology, 1. Granites, by Thomas S. Hart * Sir William Crookes * Summaries and Notes from the Mining Journals * Students' Association * Sports * The Bush Assayer * Solubility of Gold-Silver Alloys in Potassium Cyanide * Correspondence Vol 2, No. 2, April 1899 * News and Notes (Smythesdale Excursion, New Buildings, A.S. Coyte, R.J. Allan) * History of the Ballarat School of Mines (Continued) * The New Students (J. Owen, A. Clayton Morrisby, A.S. Atkin, J. Alexander Reid, Alfred G. Johnston, L. Lowe, F.H. Dalton, W.M. Robertson, A. Hacke, H.L. Giles, W. Martin, E. Walshe, H.L. Krause, R. Sawyer) * Berringa by Oh'E Jay * Summaries and Notes from the Mining Journals * Mount Magnet to Victoria - A Long Bicycle Trip * 1898 Examination returns * Sports Vol 2, No. 3, May 1899 * Technical Education and the Proposed Affiliation of the Schools of Mines with the Melbourne University. * Laying of the Foundation Stone of the New Classrooms (now Administration Building). Alexander J. Peacock * News and Notes (Past Students - A.S. Lilburn, J.W. Sutherland, J. Richardson, E. Prendergast, J. Wallace, J. Kidd, J. Lake, Mathew Thompson), Coolgardie Exhibition. * Trip to Lal Lal * Students' Association * Summaries and Notes from the Mining Journals * Professor Henry Louis on Mining Education * Corrections Used in Chaining by C.W. Adams * The Black Horse Cyanide Plant * Sports * Completed List of 1898 Examinations Vol 2, No. 4, June 1899 * News and Notes * The Education Problem by D.N. McLean * A Few Hints on Histological Technique by Emil Gutheil * Summaries and Notes from the Mining Journals * Students' Association * A Visit to the Skipton Caves (Mount Widdern, Ormand Hill, volcano, Emu Creek, Mount Kinross, Mount Elephant, Mount Vite Vite, Mount Kinross, Mount Hamiston) * Mount Magnet To Victoria (cont) * The New Engines at the Ballarat Woollen Mills - includes image of the Compound 700 H.P. Engines constructed for the Ballarat Woollen Mills by Austral Otis Company and consulting engineers Monash and Anderson. * Sports * Original Poetry * Correspondence Vol 2, No. 5, July 1899 * News and Notes (E. Byron Moore, Visit to Britannia Gold Mine, J. Bryant, Visit to Last Chance Mine) * A Few Hints on Histological Technique (cont) by Emil Gutheil * Summaries and Notes from the Mining Journals * Professor Alfred Mica Smith (includes image) * Notes on Victorian Geology Part 2 The Trappean Rocks, by Thomas Hart * Origin of Diamonds * Hydraulic Mining by A.E.C. Kerr * Volcanoes by F.G. Bonney * Analytical Chemistry Notes by Daniel Walker * Some Things Out To Do * Sports * Correspondence Vol 2, No. 6, August 1899 *Summaries and notes from the Mining Journals * Some Regulations of the Academy of Mines at Freiberg * A visit to Mt Lyell Smelters * Professor Gilbert J. Dawbarn (includes image) * Air compressor and Transmission of Power by Compressed air by A.E.C. Kerr * Chemistry Notes by Daniel Walker * Mineralogical Notes, Ballarat by Thomas S. Hart * Kalgurli Gold Mines, W.A. * OUr New Lab Vol 2., No 7, September 1899 * Summaries and Notes from the Mining Journals * Some recent Steam Plants at Bendigo by Gilbert Dawbarn * Professor Thomas Stephen Hart (includes image) * Students Association * Notes on Victorian Geology by Thomas Hart * Centrifugal Pumps * A New Chum's Experience by E.M. Weston Vol 2., No 8, October 1899 * The institute of Chemistry Examinations * A New Method of Qualitative Chemical Analysis by Emil Gutheil * Steam Engine Valves and Valve-Gears by Gilbert Dawbarn * Daniel Walker (includes image) * Notes on Victorian Geology by Thomas Hart * Cyaniding Cripple Creek Tellurides (Metallic Extraction Company) * Notes on Two Ballarat Gravel Pumping Plants, G.A. Wilberforce (Eureka Jennings Co and Yarrowee Sluicing Co) * History of the School of Mines (concluded) Vol 3., No 1, March 1900 * A Journey from Natal to Mashomaland with the British Police * A Plea for Research * New Caledonia by C.A.M. Deane * Notes of Victorian Geology - Lower Palaeoroic Rocks by Thomas Hart * Mt Bischoff Mine and Mill * Summaries and Notes from the Mining Journals * Things we Eat and Drink * Farewell to A.S. Coyte Vol 3., No 1, March 1900 * Mining Education * Model Locomotive made by the apprentices of the Phoenix Foundry, p2 * Glimpses of Rhodesian Police Camp Life * New Caledonia (continued) * Summaries from the Mining and Engineering Journals * Boot and Saddle Vol 3., No 3, May 1900 * A Students' Common Room * Geological Excursion to Hardie's Hill * Notes on Victorian Geology by Thomas Hart * The Planet Venus by John Brittain * Summaries and Notes from the Australian Mining Standard * The Assay Ton * Zeehan Smelters * Electrical Notes by Ohe Jay * Trop of the Cricket Club to Stawell * Students' Association * Solid Hydrogen Vol 3., No 4, June 1900 * The Minister of Mines on Mining Education (Minister A.R. Outtrim) * Lal Lal Geology Trip (Thomas Hart) * Rifle Club now defunct, pg 3 * A Contribution to the Mining Geology of Kalgoorlie, W.A. by Ferdinand Krause (includes cross sections) (Wood's Point, Rand, Johannesburg, South Africa, Gaffney's Creek, Walhalla, Shady Creek, Sago Hill at Cardigan, Bunbury) * Summaries and Notes from the Australian Mining Standard (Buninyong Estate Mine) * Monthly Progress Reports of the Geological Survey * Electrical Notes by John M Sutherland (Telagraphone, phonograph, telephone receiver) * Students' Theatre Party (Gordon Todd, Ohe Jaeger, C.S. Wakley) * Opening of the New Buildings - Ministerial Speeches (Outtrim, W.H. Irvine, New Mining Laboratory, Old Chemistry Building, Battery, Model Mine) * Students' Association * Relief of Mafeking * A Critic Criticised * Things We Eat and Drink by Ohe Jay - Oatmeal, Coffee and Cocoa. Vol 3., No 5, July 1900 * Research * Adelaide Varsity Students at Ballarat * The Manchester-Liverpool Mono Railway * Students Association * *A Contribution to the Mining Geology of Kalgoorlie, W.A. by Ferdinand Krause (continued) (includes cross-sections) * Motive Power, address by Charles A. Parsons * Summaries and Notes from the Australian Mining Standard * Sugar Manufacturing by Sugna * Great Creswick Hydraulic Sluicing Plant (THomas Hart, Ballarat School of Mines Mining Class visit) * Reminiscences of a Students Life in Germany * Football - Ballarat School of Mines v Geelong Grammar School (Australian Rules Football) Vol 3., No 6, August 1900 * Cheap Mine Management * Library * Bendigo School of Mines, pg 3 * Notes on Ore Dressing by T, Vincent, Manager The Zeehan (Tas) Silver-Lead Mines Ltd) * Motive Power * Notes on Broken Hill - Its Mines and Minerals by J. Williams * The Concert * Summaries and Notes from the Australian Mining Standard * The Dandy Duke's Dreadful Demise * The Road Race Vol 3., No 7, September 1900 * Michaelmas Excursion (Melbourne University, Prof Kernot, Applied Mechanics) * Injury to School Property * Return of E. Ditchburn (Boer War) * Mt William Gold-Field visit, pg 3 * The Stoping of Wide Lodes by J.V. Lake (includes cross sections) * Summaries of Notes from the Australian Mining Standard * Notes on Broken Hill Part 2- Its Mines and Minerals by W.J. Williams * Motive Power from the Waves * Electrical Notes * Some Account of Italian Mining (Sarinia, Sicily, Peidmont, Lombardia) by Candido Maglione * Students Association * Should Women Have the Vote by Frank Bessemeres * The School Theatre Parly * Past Students * Poetry * Football * Surveying Rules Vol 3., No 8, October 1900 * Ballarat School of Mines Associateship * An Engineering Laboratory * Students' Practical Work * Notes on Broken Hill Part 3 by W.J. Williams * The Lake View Consols by F.S. Earp - Battery Treatment of Sulpo-Telluride Ore * Neglected Mineral Fields - Eurowie and Warrata * A Glimpse Ahead * News and Notes * A.W. G. McPherson, Boer War * Students Association * Ballarat School of Mines Melbourne Excursion to the Government Electric Lighting Station, Austral-Otis Co, Working Mens College * Ballarat School of Mines Concert in Aid of Soldiers Statue Balance Sheet * Football * Cricket Vol 3., No 8b, November 1900 * Position of the Ballarat School of Mines with Regards to Mining Education * Age Limit * Entrance Examination * Presentation t0 Professor Alfred Mica Smith * Image of a Group of Old Ballarat School of Mines Students in Coolgardie and Kalgoorlie. * Students Association Vol 4., No 1, March 1901 * Espirit De Corps * A few Notes on the Testing of Explosives * Round About Inverell, NSW by F. and J. Mawl * On the Choice of Drawing Instruments * Summaries and Notes From the Technical Journals * Annual Examinations 1900 * New Students * Sporting Notes * The Vale of Coolgardie Mine, Bonnievale, W.A. by G. Stephen Hart * News and Notes (Kerr Grant, C.L. Nash, R. Gordon Todd, Vial) * Editorial Notices Vol 4., No 2, Second Term 1901 * The Metallurgical Treatment of Sulpho-Telluride Ores by L.W. Grayson * Some Metallurgical Difficulties of Aluminium * Diehl's Sulphide Process by A.E. C. Kerr * A Californian Gold Mine by A.E. C. Kerr * New Express Locomotives for the Victorian Government (Phoenix Foundry) * An Excursion to Geelong (Electric Light and Traction Company of Australia) * The Linkenback Table for our New Mining Laboratory (Humboldt Company of Colgne) * Death of Thomas Bath * The Late Alfred G. Johnson (Boer War) * An Introduction to Natural Science by Emil Gutheil * The First Annual School Sports Meeting * Concert in Aid of Magazine Funds * The Men That Made the Concert (C.E. Denniston, W.H. Chandler, Mr White, William Litte Jnr, Marriott, Giles McCracken) * Sports * News and Notes Vol 4., No 2, Third Term 1901 * Bagging-Up - A Sketch * Concentration of Difficult Silver-Lead Ores * Estimation of Chlorine, Bromine and Iodine by D. Runting * Summaries of Notes from teh technical Journals * Notes on the Use and Care of Platinum Ware Common Sense * The Machinery at the Tasmania Gold Mine, Beaconsfield, Tasmania * Mining at Walhalla - The Long Tunnel Mine * Past Students * Mapping our of Agricultural Areas, etc, In Dense Vine Lands, North Queensland by R.A. Suter * News and Notes * Concert Balance Sheet e.m. weston, robert brough smyth, mcdougall, bruce, charles burbury, harrie wood, graham j. hopwood, emil gutheil, daniel walker, thomas hart, thomas stephen hart, m. hacker, schnitzler, f.a., ditchfield, l.h, alfred e.c. kerr, charles harvey, campbell, joseph bryant, campbell & ferguson, gilbert j. dawburn, irving, g.b., kerr, a.e.c., john walter sutherland, william robertson, herbert l. krause, alfred mica smith, binh pham, crosbie, d. jack, ditchburn, j., james hiscock, alfred johnston, reid, j.a., kidd, john, james bonwick, james, j.p, overall, d, e.h salmon, gaynor marquand, williams, w.w., williams, william, deane, c.m., vincent, tom, phillips, g.e., hart, d.w., jarnail suingh, rowlands, e., ferdinand m. krause,, easterby, f.l, parsons, r.g., partington, j.r., vial, s.b., meadows, h, atkins, arthur, john braisted burdekin, w.h. corbould, ditchburn, john, hill, john, otto e. jager, mcpherson, g.t, nicholls, c, thom, j.m., crafter, stewart, john brittain, peter lalor, hardy - commissioner, thomas bath, alf johnston, charles campbell, nash, llewellyn, watson, m.a, gardener, eddie, adamson, s.g, alford, l.c, allen, r.j, arthur, d.w.b., burge, a., willia, cairncross, cooper, i, maurice osric copland, maurice copland, dickinson, s., doepel, dunstan, john, loveday dunstan, eeles, terri, flegeltaub, israel, fletcher, a, fyrar, peter, kerr grant, w.kerr, green, gary, betty harris, harris, c.m., hay, a.l., hearn, hill, martin, james, david, johnston, alfred g, kilner, marion, kingston, thomas, lewin, f.c.k., lilburne, arthur m, linahan, colin, macready, w.h, major birlefco, markwald, henry, mccaffrey, mcfarlane, kaye, mciver, s.k, mellins, b, morton, felicity, w. kenneth moss, ken moss, nash, c.w., nash, neville, nickolls, berkeley, osborne, percy, philp, e., playford, william, reid, e, roberts, gordon, ross, f.c., royce, phillip, sawyer, basil, stewart, r.c., todhunter, i, vaisey, a., vincent, john, vinden, sue, wakley, cecil, watt, james, westcott, lewis, charles w. whyte,, vial, s browning, ballarat school of mines students in coolgardie and kalgoorlie, coolgardie, kalgoorlie, claude maitland, a.l. hay, a.s. lilburne, latham watson, arthur kildahl, thomas copeland, f.a. moss, w.a. hearman, cardoc james, alexander fraser, e.o. watt, g.m. roberts, j.j. dunstan, h.v. moss, j.a. hill,, john dunstan, c.m. harris, william h. corbould, j.w. sutherland, ballarat photographic club, ballarat field naturalists club, ballarat field club and science society, photography, geology, excursions, last chance mine, tasmania gold mine, beaconsfield, tasmania, rand, south africa, mount lyell, ballarat school of mines student excursion to mount lyell, h.l. krause, ferdinand krause, krause, hardie's hill, hardie's hill excursion, lal lal, lal lal excursion, lal lal geology excursion, smythesdale, smythesdale excursion, soudan, south african miners, south star mines, wynne and tregurtha battery, ananconda copper mining, arizona copper mining, boiler plates, british guinea, butte copper smelter, daylesford geology camp, daylesford excursion, diehl process, electric power house ballarat, electric pumps, geelong rope factory, gympie, golden horseshoe estate, c johnstone, jack nichol, c. macgennis, alec saunders, alfred g. johnstone, graeme jolly, william purdie, john mann, maxwell l gaunt, sale school of mines, freiberg school of mines, schools of mines, railway locomotive -
 Federation University Historical Collection
Federation University Historical CollectionPhotograph - Photograph - black and white, Ballarat Junior Technical School Cadet Team, 1916, 1916
... . Major K.D. Norman C. of E. A.I.F. officiated. The grave.... Major K.D. Norman C. of E. A.I.F. officiated. The grave ...Francis Davis was the only former student of the Ballarat Junior Technical School to die during World War One. "Francis Davis was accorded a full military funeral, firing party, bugler and pallbearers. The coffin was draped in the Union Jack and surmounted in several beautiful wreathes sent from his brother 2/A.M. E.H. Davis (A.F.C Leighterton), officer of the A.F.C. Leighterton, Gloucester, Cadets of A.F.C. and many other personal friends of the deceased. The "Last Post" was sounded at the graveside, and the Rev. Major K.D. Norman C. of E. A.I.F. officiated. The grave was to be turfed and an oak cross erected by the A.I.F. London. Administrative Headquarters A.I.F. London were represented at the funeral. (http://naa12.naa.gov.au/scripts/Imagine.asp?B=1858392, accessed 24 January 2014.) According to Neil Leckie, Manager of the Ballarat Ranger Military Museum: * Originally 12 – 14 year olds went to Junior Cadets attached to their school. * From age 14 – 17 they were Senior Cadets attached to the local militia unit. * After 1 July of the year a Cadet turned 18, the Cadet left the Senior Cadets and became a member of the Citizen Military Force. * In October 1918 the AIF, Militia and Cadets were renamed to give some connection to the AIF battalion raised in the area. Ballarat saw: 8th Australian Infantry Regiment comprising: * 8th Battalion AIF renamed 1st Battalion 8th Australian Infantry Regiment * 70th Infantry Militia renamed 2nd Battalion 8th Australian Infantry Regiment * 70th Infantry Cadets renamed 3rd B, 8th Australian Infantry. 39th Australian Infantry Regiment comprising: * 39th Battalion AIF renamed 1st Battalion 39th Australian Regiment * 71st Infantry Militia renamed 2nd Bn, 39th Australian Infantry Regiment * 71st Infantry Cadets renamed 3rd Bn, 39th Australian Infantry Regiment Prior to the reorganisation in 1918 the 18th Brigade was the 70th, 71st and 73rd Infantry. It is thought that the 18th Brigade Cadet units in 1920 were those that came from the old: * 69th Infantry (Geelong/Queenscliff) * 70th Infantry (Ballarat/Colac) * 71st Infantry (Ballarat West) * 72nd Infantry Warrnambool) * 73rd Infantry (NW Vic) The next name change came in 1921!Black and white photograph of a group of school boys in army uniform. They are members of the Ballarat Junior Technical School Cadets. Back Row: D.O. Taylor, Albert E. Williams, B. Burrows, J.B. Hobba Standing: Francis Davis, Miller, A. Burge, P. [Peter] Chatham. J. Minster, H. Witter, H. Siemering Kneeling: T.G. Wasley, Alan Riley, A.H. Hoskin. N.C. Carmichael, Harold G. Wakeling, T. Rees, W.H. Shattock, F.N. Gibbs Front: S.J. Chambers, F.J. Procter, Charles H. Beanland francis davis, frank davis, ballarat junior technical school cadets, cadets, d.o. taylor, albert e. williams, b. burrows, j.b. hobba, miller, a. burge, p. chatham, j. minster, h. witter, h. siemering, t.g. wasley, a. riley, a.h. hoskin, n.c. carmichael, harold wakeling, t. rees, w.h. shattock, f.n. gibbs, s.j. chambers, f.j. procter, charles h. beanland, alan riley -
 Federation University Historical Collection
Federation University Historical CollectionPhotograph - Colour, Memorial to Francis Davis in the grounds of Federation University, SMB Campus, 2012, 20/09/2012
... . Major K.D. Norman C. of E. A.I.F. officiated. The grave..." was sounded at the graveside, and the Rev. Major K.D. Norman C. of E ...Francis Gordon Davis was born in Ballarat on 09 August 1899. He is the only former student of the Ballarat Junior Technical School who was killed on service during World War One. Davis enlisted into the Australian Flying Corps, Laverton, on 12 April 1918 at which time he was 18 and 8 months and served as a second class Air Mechanic. His service number was 3310. He died accidentally from shock resulting from an accident resulting from skidding a Leyland Motor Lorry at Leighterton, Tetbury, Gloucester, England on 28 January 1919 and is buried in Grave 6 in the Soldiers Corner of the Leighterton Cemetery. Francis Davis was accorded a full military funeral, firing party, bugler and pallbearers. The coffin was draped in the Union Jack and surmounted in several beautiful wreathes sent from his brother 2/A.M. E.H. Davis (A.F.C Leighterton), officer of the A.F.C. Leighterton, Gloucester, Cadets of A.F.C. and many other personal friends of the deceased. The "Last Post" was sounded at the graveside, and the Rev. Major K.D. Norman C. of E. A.I.F. officiated. The grave was to be turfed and an oak cross erected by the A.I.F. London. Administrative Headquarters A.I.F. London were represented at the funeral. (http://bih/index.php/Francis_G._Davis) In June 1922 Alfred Davis, the father of Francis Davis, planted a tree in the grounds of the Ballarat Junior Technical School in honour of hos son. It was the first tree of six planted in the grounds of the Ballarat School of Mines on Arbor Day 1922. Speaking of the planting of the tree by Mr Davis the Chief Secretary (Mr M. Baird M.L.A.), said he trusted the memory would ever remain green at the school. Had he and others not given their lives nothing that we could have done to-day could have retrieved the time. Australians had indeed done splendidly, but they should take a wider outlook than Australia, and reading the history of the Genoa Conference he had been struck by what had been done ... We should honor such men as he in whose memory that tree was planted, and the schools that sent them out to fight for us. He hoped the empire would always be able to produce such men, so that the Empire would always be able to lead the World's struggle for the benefit of humanity. The last post was then sounded by Mr. H. Green. ... (Ballarat Courier, 19 June 1922)A number of photographs of a tree and marble plaque in the grounds of the Ballarat School of Mines. It was a memorial to Francis Davis, a former student of the Ballarat Junior Technical School, who died on active service during World War One.ballarat school of mines, ballarat junior secondary school, world war one, memorial, marble plaque, marble memorial, tree, memorial tree, davis, francis davis -
 Flagstaff Hill Maritime Museum and Village
Flagstaff Hill Maritime Museum and VillageDomestic object - Plate
The Process of Making Pottery Decorating, Firing, Glazing, Making, Technical There is a rhythm and flow to clay. It can’t be done all at once! Even the making process! It can take weeks to get everything done, especially if you can only work on your pottery once a week! Even though we have three hour classes, it’s often just not enough time! Here is an overview of some of the processes so you have a bit more grasp on some of the technical stuff! Step One – Design There are SO many ideas out there for making stuff in clay! From delicate porcelain jewellery, through to heavy sculptural work and everything in between. Deciding your direction is sometimes not that easy – when you first start, try everything, you will naturally gravitate to the style that you enjoy! The options and variations are endless and can get a wee bit overwhelming too! Check in with me before you start to ensure your ideas will work, what order you might do things, how you could achieve the look you are seeking and any other technical data required! Step Two – Making Clay is thixotropic. This means that as you work with it, the clay first gets sloppier and wetter, before is begins to dry in the atmosphere. For most things, you simply can’t do all parts of the project at once. An example of work order might look like: Get last weeks work out from the shelves Prepare clay for today’s work – roll your clay, prepare balls for throwing, make the first stage of a pinch pot) Clean up last week’s work and put it on the shelf for bisque firing Check that you have any glazing to do – and do enough of it that you will have time to finish your main project Do the next step of your next project – there might be a further step that can’t be complete immediately, in that case, wrap your work well and put onto the shelves. Letting your work rest for a while can really help keep your work clean and professional looking. Many things require bagging under plastic to keep it ready for work the next week – put your name on the outside of the bag so you can find your work easily. We have stickers and markers. Consider how you want to decorate your work – coloured slip can be applied at a fairly wet stage (remembering that it will make your work even wetter!). Trying to apply slip to dry clay won’t work! If you want to do sgraffito – you will need to keep the work leather hard (a state of dryness where you can still work the clay with a little effort and a little water and care). Step Three – Drying Most of the time your work can go into the rack uncovered to let it dry out for the following week. If you want to continue forming or shaping you will need to double bag your work – put your work on a suitable sized bat and put the bat in a bag so the base of the bag is under the bat, then put another bag over the top of the work and tuck the top of the bag under the bat. If you want to trim (or turn) your thrown work the following week, it should also be double bagged. If your work is large, delicate, or of uneven thicknesses, you should lightly cover your work for drying. When considering the drying process, bare in mind the weather, humidity and wind! The hotter and dryer, the faster things dry and work can dry unevenly in the shelves – this can lead to cracking – another time to lightly cover your work for drying. Step Four – Trimming and Cleaning Up Your work is dry! It is called greenware now and it is at it’s most fragile! Handle everything with two hands. I often refer to soft hands – keep everything gentle and with your fingers spread as much as possible. Try to not pick up things like plates too much, and always with both hands! Before your work can be bisque fired it should be “cleaned up”. You work won’t go into the kiln if it has sharp edges – when glazed, sharp edges turn into razor blades! Use a piece of fly wire to rub the work all over – this will scratch a little so be light handed. Use a knife or metal kidney to scrape any areas that require a bit more dynamic treatment than the fly wire offers! Finally, a very light wipe over with a slightly damp sponge can help soften and soothe all of your edges and dags! Trimming thrown work: If you are planning to trim (or turn) your thrown work (and you should be), make sure you bag it well – your work should be leather hard to almost dry for easiest trimming. Use this step to finish the work completely – use a metal kidney to polish the surface, or a slightly damp sponge to give a freshly thrown look. Wipe the sponge around the rim after trimming, and check the inside of the pot for dags! Trimming slip cast work: Usually I will trim the rims of your work on the wheel the following day to make that stage easier, however you will still need to check your work for lumps and bumps. Last but not least – check that your name is still clearly on the bottom of your work. Step Five – Bisque Firing When the work is completely dry it can go into the bisque kiln. The bisque kiln is fired to 1000°C. This process burns off the water in the clay as well as some of the chemically bound water. The structure of the clay is not altered that much at this temperature. Inside the bisque kiln, the work is stacked a little, small bowl inside a larger bowl and onto a heavy plate. Smaller items like decorations or drink coasters might get stacked several high. Consideration is paid to the weight of the stack and shape of the work. A bisque kiln can fire about one and a half times the amount of work that the glaze kiln can fire. The firing takes about 10 hours to complete the cycle and about two days to cool down. Once it has been emptied the work is placed in the glaze room ready for you to decorate! Step Six – Glazing Decorating your work with colour can be a lot of fun – and time consuming! There are three main options for surface treatment at this stage: Oxide Washes Underglazes Glazes Washes and underglazes do not “glaze” the work – It will still need a layer of glaze to fully seal the clay (washes don’t need glaze on surfaces not designed for food or liquid as they can gloss up a little on their own). Underglazes are stable colourants that turn out pretty much how they look in the jar. They can be mixed with each other to form other colours and can be used like water colours to paint onto your work. Mostly they should have a clear glaze on top to seal them. Oxides are a different species – the pink oxide (cobalt) wash turns out bright blue for instance. They don’t always need a glaze on top, and some glazes can change the colour of the wash! The glazes need no other “glaze” on top! Be careful of unknown glaze interactions – you can put any combination of glaze in a bowl or on a plate, but only a single glaze on the outside of any vertical surface! Glazes are a chemical reaction under heat. We don’t know the exact chemicals in the Mayco glazes we use. I can guess by the way they interact with each other, however, on the whole, you need to test every idea you have, and not run the test on a vertical surface! Simply put, glaze is a layer of glass like substance that bonds with the clay underneath. Clay is made of silica, alumina and water. Glaze is made of mostly silica. Silica has a melting point of 1700°C and we fire to 1240°C. The silica requires a “flux” to help it melt at the lower temperature. Fluxes can be all sorts of chemicals – a common one is calcium – calcium has a melting point of 2500°C, however, together they both melt at a much lower temperature! Colourants are metal oxides like cobalt (blue), chrome (green through black), copper (green, blue, even red!), manganese (black, purple and pink) iron (red brown), etc. Different chemicals in the glaze can have dramatic effects. for example, barium carbonate (which we don’t use) turns manganese bright pink! Other elements can turn manganese dioxide brown, blue, purple and reddish brown. Manganese dioxide is a flux in and of itself as well. So, glazes that get their black and purple colours, often interact with other glazes and RUN! Our mirror black is a good example – it mixes really well with many glazes because it fluxes them – causes them to melt faster. It will also bring out many beautiful colours in the glazes because it’s black colouring most definitely comes from manganese dioxide! Glaze chemistry is a whole subject on it’s own! We use commercial Mayco glazes on purpose – for their huge range of colour possibilities, stability, cool interactions, artistic freedom with the ability to easily brush the glazes on and ease of use. We currently have almost 50 glazes on hand! A major project is to test the interactions of all glazes with each other. That is 2,500 test tiles!!!! I’m going to make the wall behind the wheels the feature wall of pretty colours! Step Seven – Glaze (Gloss or sometimes called “Glost”) Firing Most of the time this is the final stage of making your creation (but not always!) The glaze kiln goes to 1240°C. This is called cone 6, or midrange. It is the low end of stoneware temperatures. Stoneware clays and glazes are typically fired at cone 8 – 10, that is 1260 – 1290°C. The energy requirement to go from 1240°C to 1280°C is almost a 30% more! Our clay is formulated to vitrify (mature, turn “glass-like”) at 1240°, as are our glazes. A glaze kiln take around 12 hours to reach temperature and two to three days to cool down. Sometimes a third firing process is required – this is for decoration that is added to work after the glaze firing. For example – adding precious metals and lustres. this firing temperature is usually around 600 – 800°C depending upon the techniques being used. There are many students interested in gold and silver trims – we will be doing this third type of firing soon! After firing your work will be in the student finished work shelves. Remember to pay for it before you head out the door! There is a small extra charge for using porcelain clay (it’s more than twice the price of regular clay), and for any third firing process! Once your work has been fired it can not turn back into clay for millennia – so don’t fire it if you don’t like it! Put it in the bucket for recycling. https://firebirdstudios.com.au/the-process-of-making-pottery/Ceramics have evolved over thousands of years.White earthenware dinner plate. Crazing evident all over.Backstamped ‘Made in England S LTD’flagstaff hill, warrnambool, shipwrecked-coast, flagstaff-hill, flagstaff-hill-maritime-museum, maritime-museum, shipwreck-coast, flagstaff-hill-maritime-village, ceramics, tableware -
 Flagstaff Hill Maritime Museum and Village
Flagstaff Hill Maritime Museum and VillageDomestic object - Plate, Johnson Bros
The Process of Making Pottery Decorating, Firing, Glazing, Making, Technical There is a rhythm and flow to clay. It can’t be done all at once! Even the making process! It can take weeks to get everything done, especially if you can only work on your pottery once a week! Even though we have three hour classes, it’s often just not enough time! Here is an overview of some of the processes so you have a bit more grasp on some of the technical stuff! Step One – Design There are SO many ideas out there for making stuff in clay! From delicate porcelain jewellery, through to heavy sculptural work and everything in between. Deciding your direction is sometimes not that easy – when you first start, try everything, you will naturally gravitate to the style that you enjoy! The options and variations are endless and can get a wee bit overwhelming too! Check in with me before you start to ensure your ideas will work, what order you might do things, how you could achieve the look you are seeking and any other technical data required! Step Two – Making Clay is thixotropic. This means that as you work with it, the clay first gets sloppier and wetter, before is begins to dry in the atmosphere. For most things, you simply can’t do all parts of the project at once. An example of work order might look like: Get last weeks work out from the shelves Prepare clay for today’s work – roll your clay, prepare balls for throwing, make the first stage of a pinch pot) Clean up last week’s work and put it on the shelf for bisque firing Check that you have any glazing to do – and do enough of it that you will have time to finish your main project Do the next step of your next project – there might be a further step that can’t be complete immediately, in that case, wrap your work well and put onto the shelves. Letting your work rest for a while can really help keep your work clean and professional looking. Many things require bagging under plastic to keep it ready for work the next week – put your name on the outside of the bag so you can find your work easily. We have stickers and markers. Consider how you want to decorate your work – coloured slip can be applied at a fairly wet stage (remembering that it will make your work even wetter!). Trying to apply slip to dry clay won’t work! If you want to do sgraffito – you will need to keep the work leather hard (a state of dryness where you can still work the clay with a little effort and a little water and care). Step Three – Drying Most of the time your work can go into the rack uncovered to let it dry out for the following week. If you want to continue forming or shaping you will need to double bag your work – put your work on a suitable sized bat and put the bat in a bag so the base of the bag is under the bat, then put another bag over the top of the work and tuck the top of the bag under the bat. If you want to trim (or turn) your thrown work the following week, it should also be double bagged. If your work is large, delicate, or of uneven thicknesses, you should lightly cover your work for drying. When considering the drying process, bare in mind the weather, humidity and wind! The hotter and dryer, the faster things dry and work can dry unevenly in the shelves – this can lead to cracking – another time to lightly cover your work for drying. Step Four – Trimming and Cleaning Up Your work is dry! It is called greenware now and it is at it’s most fragile! Handle everything with two hands. I often refer to soft hands – keep everything gentle and with your fingers spread as much as possible. Try to not pick up things like plates too much, and always with both hands! Before your work can be bisque fired it should be “cleaned up”. You work won’t go into the kiln if it has sharp edges – when glazed, sharp edges turn into razor blades! Use a piece of fly wire to rub the work all over – this will scratch a little so be light handed. Use a knife or metal kidney to scrape any areas that require a bit more dynamic treatment than the fly wire offers! Finally, a very light wipe over with a slightly damp sponge can help soften and soothe all of your edges and dags! Trimming thrown work: If you are planning to trim (or turn) your thrown work (and you should be), make sure you bag it well – your work should be leather hard to almost dry for easiest trimming. Use this step to finish the work completely – use a metal kidney to polish the surface, or a slightly damp sponge to give a freshly thrown look. Wipe the sponge around the rim after trimming, and check the inside of the pot for dags! Trimming slip cast work: Usually I will trim the rims of your work on the wheel the following day to make that stage easier, however you will still need to check your work for lumps and bumps. Last but not least – check that your name is still clearly on the bottom of your work. Step Five – Bisque Firing When the work is completely dry it can go into the bisque kiln. The bisque kiln is fired to 1000°C. This process burns off the water in the clay as well as some of the chemically bound water. The structure of the clay is not altered that much at this temperature. Inside the bisque kiln, the work is stacked a little, small bowl inside a larger bowl and onto a heavy plate. Smaller items like decorations or drink coasters might get stacked several high. Consideration is paid to the weight of the stack and shape of the work. A bisque kiln can fire about one and a half times the amount of work that the glaze kiln can fire. The firing takes about 10 hours to complete the cycle and about two days to cool down. Once it has been emptied the work is placed in the glaze room ready for you to decorate! Step Six – Glazing Decorating your work with colour can be a lot of fun – and time consuming! There are three main options for surface treatment at this stage: Oxide Washes Underglazes Glazes Washes and underglazes do not “glaze” the work – It will still need a layer of glaze to fully seal the clay (washes don’t need glaze on surfaces not designed for food or liquid as they can gloss up a little on their own). Underglazes are stable colourants that turn out pretty much how they look in the jar. They can be mixed with each other to form other colours and can be used like water colours to paint onto your work. Mostly they should have a clear glaze on top to seal them. Oxides are a different species – the pink oxide (cobalt) wash turns out bright blue for instance. They don’t always need a glaze on top, and some glazes can change the colour of the wash! The glazes need no other “glaze” on top! Be careful of unknown glaze interactions – you can put any combination of glaze in a bowl or on a plate, but only a single glaze on the outside of any vertical surface! Glazes are a chemical reaction under heat. We don’t know the exact chemicals in the Mayco glazes we use. I can guess by the way they interact with each other, however, on the whole, you need to test every idea you have, and not run the test on a vertical surface! Simply put, glaze is a layer of glass like substance that bonds with the clay underneath. Clay is made of silica, alumina and water. Glaze is made of mostly silica. Silica has a melting point of 1700°C and we fire to 1240°C. The silica requires a “flux” to help it melt at the lower temperature. Fluxes can be all sorts of chemicals – a common one is calcium – calcium has a melting point of 2500°C, however, together they both melt at a much lower temperature! Colourants are metal oxides like cobalt (blue), chrome (green through black), copper (green, blue, even red!), manganese (black, purple and pink) iron (red brown), etc. Different chemicals in the glaze can have dramatic effects. for example, barium carbonate (which we don’t use) turns manganese bright pink! Other elements can turn manganese dioxide brown, blue, purple and reddish brown. Manganese dioxide is a flux in and of itself as well. So, glazes that get their black and purple colours, often interact with other glazes and RUN! Our mirror black is a good example – it mixes really well with many glazes because it fluxes them – causes them to melt faster. It will also bring out many beautiful colours in the glazes because it’s black colouring most definitely comes from manganese dioxide! Glaze chemistry is a whole subject on it’s own! We use commercial Mayco glazes on purpose – for their huge range of colour possibilities, stability, cool interactions, artistic freedom with the ability to easily brush the glazes on and ease of use. We currently have almost 50 glazes on hand! A major project is to test the interactions of all glazes with each other. That is 2,500 test tiles!!!! I’m going to make the wall behind the wheels the feature wall of pretty colours! Step Seven – Glaze (Gloss or sometimes called “Glost”) Firing Most of the time this is the final stage of making your creation (but not always!) The glaze kiln goes to 1240°C. This is called cone 6, or midrange. It is the low end of stoneware temperatures. Stoneware clays and glazes are typically fired at cone 8 – 10, that is 1260 – 1290°C. The energy requirement to go from 1240°C to 1280°C is almost a 30% more! Our clay is formulated to vitrify (mature, turn “glass-like”) at 1240°, as are our glazes. A glaze kiln take around 12 hours to reach temperature and two to three days to cool down. Sometimes a third firing process is required – this is for decoration that is added to work after the glaze firing. For example – adding precious metals and lustres. this firing temperature is usually around 600 – 800°C depending upon the techniques being used. There are many students interested in gold and silver trims – we will be doing this third type of firing soon! After firing your work will be in the student finished work shelves. Remember to pay for it before you head out the door! There is a small extra charge for using porcelain clay (it’s more than twice the price of regular clay), and for any third firing process! Once your work has been fired it can not turn back into clay for millennia – so don’t fire it if you don’t like it! Put it in the bucket for recycling. https://firebirdstudios.com.au/the-process-of-making-pottery/Ceramics have evolved over thousands of years.A white earthenware side plate with a gadroon edge. Has water marks and chips on front.‘Johnson Bros England Reg No 15587’flagstaff hill, warrnambool, shipwrecked-coast, flagstaff-hill, flagstaff-hill-maritime-museum, maritime-museum, shipwreck-coast, flagstaff-hill-maritime-village, johnson bros, ceramics, tableware -
 Flagstaff Hill Maritime Museum and Village
Flagstaff Hill Maritime Museum and VillageDomestic object - Plate, Alfred Meakin
The Process of Making Pottery Decorating, Firing, Glazing, Making, Technical There is a rhythm and flow to clay. It can’t be done all at once! Even the making process! It can take weeks to get everything done, especially if you can only work on your pottery once a week! Even though we have three hour classes, it’s often just not enough time! Here is an overview of some of the processes so you have a bit more grasp on some of the technical stuff! Step One – Design There are SO many ideas out there for making stuff in clay! From delicate porcelain jewellery, through to heavy sculptural work and everything in between. Deciding your direction is sometimes not that easy – when you first start, try everything, you will naturally gravitate to the style that you enjoy! The options and variations are endless and can get a wee bit overwhelming too! Check in with me before you start to ensure your ideas will work, what order you might do things, how you could achieve the look you are seeking and any other technical data required! Step Two – Making Clay is thixotropic. This means that as you work with it, the clay first gets sloppier and wetter, before is begins to dry in the atmosphere. For most things, you simply can’t do all parts of the project at once. An example of work order might look like: Get last weeks work out from the shelves Prepare clay for today’s work – roll your clay, prepare balls for throwing, make the first stage of a pinch pot) Clean up last week’s work and put it on the shelf for bisque firing Check that you have any glazing to do – and do enough of it that you will have time to finish your main project Do the next step of your next project – there might be a further step that can’t be complete immediately, in that case, wrap your work well and put onto the shelves. Letting your work rest for a while can really help keep your work clean and professional looking. Many things require bagging under plastic to keep it ready for work the next week – put your name on the outside of the bag so you can find your work easily. We have stickers and markers. Consider how you want to decorate your work – coloured slip can be applied at a fairly wet stage (remembering that it will make your work even wetter!). Trying to apply slip to dry clay won’t work! If you want to do sgraffito – you will need to keep the work leather hard (a state of dryness where you can still work the clay with a little effort and a little water and care). Step Three – Drying Most of the time your work can go into the rack uncovered to let it dry out for the following week. If you want to continue forming or shaping you will need to double bag your work – put your work on a suitable sized bat and put the bat in a bag so the base of the bag is under the bat, then put another bag over the top of the work and tuck the top of the bag under the bat. If you want to trim (or turn) your thrown work the following week, it should also be double bagged. If your work is large, delicate, or of uneven thicknesses, you should lightly cover your work for drying. When considering the drying process, bare in mind the weather, humidity and wind! The hotter and dryer, the faster things dry and work can dry unevenly in the shelves – this can lead to cracking – another time to lightly cover your work for drying. Step Four – Trimming and Cleaning Up Your work is dry! It is called greenware now and it is at it’s most fragile! Handle everything with two hands. I often refer to soft hands – keep everything gentle and with your fingers spread as much as possible. Try to not pick up things like plates too much, and always with both hands! Before your work can be bisque fired it should be “cleaned up”. You work won’t go into the kiln if it has sharp edges – when glazed, sharp edges turn into razor blades! Use a piece of fly wire to rub the work all over – this will scratch a little so be light handed. Use a knife or metal kidney to scrape any areas that require a bit more dynamic treatment than the fly wire offers! Finally, a very light wipe over with a slightly damp sponge can help soften and soothe all of your edges and dags! Trimming thrown work: If you are planning to trim (or turn) your thrown work (and you should be), make sure you bag it well – your work should be leather hard to almost dry for easiest trimming. Use this step to finish the work completely – use a metal kidney to polish the surface, or a slightly damp sponge to give a freshly thrown look. Wipe the sponge around the rim after trimming, and check the inside of the pot for dags! Trimming slip cast work: Usually I will trim the rims of your work on the wheel the following day to make that stage easier, however you will still need to check your work for lumps and bumps. Last but not least – check that your name is still clearly on the bottom of your work. Step Five – Bisque Firing When the work is completely dry it can go into the bisque kiln. The bisque kiln is fired to 1000°C. This process burns off the water in the clay as well as some of the chemically bound water. The structure of the clay is not altered that much at this temperature. Inside the bisque kiln, the work is stacked a little, small bowl inside a larger bowl and onto a heavy plate. Smaller items like decorations or drink coasters might get stacked several high. Consideration is paid to the weight of the stack and shape of the work. A bisque kiln can fire about one and a half times the amount of work that the glaze kiln can fire. The firing takes about 10 hours to complete the cycle and about two days to cool down. Once it has been emptied the work is placed in the glaze room ready for you to decorate! Step Six – Glazing Decorating your work with colour can be a lot of fun – and time consuming! There are three main options for surface treatment at this stage: Oxide Washes Underglazes Glazes Washes and underglazes do not “glaze” the work – It will still need a layer of glaze to fully seal the clay (washes don’t need glaze on surfaces not designed for food or liquid as they can gloss up a little on their own). Underglazes are stable colourants that turn out pretty much how they look in the jar. They can be mixed with each other to form other colours and can be used like water colours to paint onto your work. Mostly they should have a clear glaze on top to seal them. Oxides are a different species – the pink oxide (cobalt) wash turns out bright blue for instance. They don’t always need a glaze on top, and some glazes can change the colour of the wash! The glazes need no other “glaze” on top! Be careful of unknown glaze interactions – you can put any combination of glaze in a bowl or on a plate, but only a single glaze on the outside of any vertical surface! Glazes are a chemical reaction under heat. We don’t know the exact chemicals in the Mayco glazes we use. I can guess by the way they interact with each other, however, on the whole, you need to test every idea you have, and not run the test on a vertical surface! Simply put, glaze is a layer of glass like substance that bonds with the clay underneath. Clay is made of silica, alumina and water. Glaze is made of mostly silica. Silica has a melting point of 1700°C and we fire to 1240°C. The silica requires a “flux” to help it melt at the lower temperature. Fluxes can be all sorts of chemicals – a common one is calcium – calcium has a melting point of 2500°C, however, together they both melt at a much lower temperature! Colourants are metal oxides like cobalt (blue), chrome (green through black), copper (green, blue, even red!), manganese (black, purple and pink) iron (red brown), etc. Different chemicals in the glaze can have dramatic effects. for example, barium carbonate (which we don’t use) turns manganese bright pink! Other elements can turn manganese dioxide brown, blue, purple and reddish brown. Manganese dioxide is a flux in and of itself as well. So, glazes that get their black and purple colours, often interact with other glazes and RUN! Our mirror black is a good example – it mixes really well with many glazes because it fluxes them – causes them to melt faster. It will also bring out many beautiful colours in the glazes because it’s black colouring most definitely comes from manganese dioxide! Glaze chemistry is a whole subject on it’s own! We use commercial Mayco glazes on purpose – for their huge range of colour possibilities, stability, cool interactions, artistic freedom with the ability to easily brush the glazes on and ease of use. We currently have almost 50 glazes on hand! A major project is to test the interactions of all glazes with each other. That is 2,500 test tiles!!!! I’m going to make the wall behind the wheels the feature wall of pretty colours! Step Seven – Glaze (Gloss or sometimes called “Glost”) Firing Most of the time this is the final stage of making your creation (but not always!) The glaze kiln goes to 1240°C. This is called cone 6, or midrange. It is the low end of stoneware temperatures. Stoneware clays and glazes are typically fired at cone 8 – 10, that is 1260 – 1290°C. The energy requirement to go from 1240°C to 1280°C is almost a 30% more! Our clay is formulated to vitrify (mature, turn “glass-like”) at 1240°, as are our glazes. A glaze kiln take around 12 hours to reach temperature and two to three days to cool down. Sometimes a third firing process is required – this is for decoration that is added to work after the glaze firing. For example – adding precious metals and lustres. this firing temperature is usually around 600 – 800°C depending upon the techniques being used. There are many students interested in gold and silver trims – we will be doing this third type of firing soon! After firing your work will be in the student finished work shelves. Remember to pay for it before you head out the door! There is a small extra charge for using porcelain clay (it’s more than twice the price of regular clay), and for any third firing process! Once your work has been fired it can not turn back into clay for millennia – so don’t fire it if you don’t like it! Put it in the bucket for recycling. https://firebirdstudios.com.au/the-process-of-making-pottery/Ceramics have evolved over thousands of years.Earthenware dessert plate, cream colour. Made by Alfred Meakin, England. Backstamped ‘Alfred Meakin England’. flagstaff hill, warrnambool, shipwrecked-coast, flagstaff-hill, flagstaff-hill-maritime-museum, maritime-museum, shipwreck-coast, flagstaff-hill-maritime-village, alfred meakin, ceramics, earthenware, kitchenware -
 Flagstaff Hill Maritime Museum and Village
Flagstaff Hill Maritime Museum and VillageDomestic object - Jug
The Process of Making Pottery Decorating, Firing, Glazing, Making, Technical There is a rhythm and flow to clay. It can’t be done all at once! Even the making process! It can take weeks to get everything done, especially if you can only work on your pottery once a week! Even though we have three hour classes, it’s often just not enough time! Here is an overview of some of the processes so you have a bit more grasp on some of the technical stuff! Step One – Design There are SO many ideas out there for making stuff in clay! From delicate porcelain jewellery, through to heavy sculptural work and everything in between. Deciding your direction is sometimes not that easy – when you first start, try everything, you will naturally gravitate to the style that you enjoy! The options and variations are endless and can get a wee bit overwhelming too! Check in with me before you start to ensure your ideas will work, what order you might do things, how you could achieve the look you are seeking and any other technical data required! Step Two – Making Clay is thixotropic. This means that as you work with it, the clay first gets sloppier and wetter, before is begins to dry in the atmosphere. For most things, you simply can’t do all parts of the project at once. An example of work order might look like: Get last weeks work out from the shelves Prepare clay for today’s work – roll your clay, prepare balls for throwing, make the first stage of a pinch pot) Clean up last week’s work and put it on the shelf for bisque firing Check that you have any glazing to do – and do enough of it that you will have time to finish your main project Do the next step of your next project – there might be a further step that can’t be complete immediately, in that case, wrap your work well and put onto the shelves. Letting your work rest for a while can really help keep your work clean and professional looking. Many things require bagging under plastic to keep it ready for work the next week – put your name on the outside of the bag so you can find your work easily. We have stickers and markers. Consider how you want to decorate your work – coloured slip can be applied at a fairly wet stage (remembering that it will make your work even wetter!). Trying to apply slip to dry clay won’t work! If you want to do sgraffito – you will need to keep the work leather hard (a state of dryness where you can still work the clay with a little effort and a little water and care). Step Three – Drying Most of the time your work can go into the rack uncovered to let it dry out for the following week. If you want to continue forming or shaping you will need to double bag your work – put your work on a suitable sized bat and put the bat in a bag so the base of the bag is under the bat, then put another bag over the top of the work and tuck the top of the bag under the bat. If you want to trim (or turn) your thrown work the following week, it should also be double bagged. If your work is large, delicate, or of uneven thicknesses, you should lightly cover your work for drying. When considering the drying process, bare in mind the weather, humidity and wind! The hotter and dryer, the faster things dry and work can dry unevenly in the shelves – this can lead to cracking – another time to lightly cover your work for drying. Step Four – Trimming and Cleaning Up Your work is dry! It is called greenware now and it is at it’s most fragile! Handle everything with two hands. I often refer to soft hands – keep everything gentle and with your fingers spread as much as possible. Try to not pick up things like plates too much, and always with both hands! Before your work can be bisque fired it should be “cleaned up”. You work won’t go into the kiln if it has sharp edges – when glazed, sharp edges turn into razor blades! Use a piece of fly wire to rub the work all over – this will scratch a little so be light handed. Use a knife or metal kidney to scrape any areas that require a bit more dynamic treatment than the fly wire offers! Finally, a very light wipe over with a slightly damp sponge can help soften and soothe all of your edges and dags! Trimming thrown work: If you are planning to trim (or turn) your thrown work (and you should be), make sure you bag it well – your work should be leather hard to almost dry for easiest trimming. Use this step to finish the work completely – use a metal kidney to polish the surface, or a slightly damp sponge to give a freshly thrown look. Wipe the sponge around the rim after trimming, and check the inside of the pot for dags! Trimming slip cast work: Usually I will trim the rims of your work on the wheel the following day to make that stage easier, however you will still need to check your work for lumps and bumps. Last but not least – check that your name is still clearly on the bottom of your work. Step Five – Bisque Firing When the work is completely dry it can go into the bisque kiln. The bisque kiln is fired to 1000°C. This process burns off the water in the clay as well as some of the chemically bound water. The structure of the clay is not altered that much at this temperature. Inside the bisque kiln, the work is stacked a little, small bowl inside a larger bowl and onto a heavy plate. Smaller items like decorations or drink coasters might get stacked several high. Consideration is paid to the weight of the stack and shape of the work. A bisque kiln can fire about one and a half times the amount of work that the glaze kiln can fire. The firing takes about 10 hours to complete the cycle and about two days to cool down. Once it has been emptied the work is placed in the glaze room ready for you to decorate! Step Six – Glazing Decorating your work with colour can be a lot of fun – and time consuming! There are three main options for surface treatment at this stage: Oxide Washes Underglazes Glazes Washes and underglazes do not “glaze” the work – It will still need a layer of glaze to fully seal the clay (washes don’t need glaze on surfaces not designed for food or liquid as they can gloss up a little on their own). Underglazes are stable colourants that turn out pretty much how they look in the jar. They can be mixed with each other to form other colours and can be used like water colours to paint onto your work. Mostly they should have a clear glaze on top to seal them. Oxides are a different species – the pink oxide (cobalt) wash turns out bright blue for instance. They don’t always need a glaze on top, and some glazes can change the colour of the wash! The glazes need no other “glaze” on top! Be careful of unknown glaze interactions – you can put any combination of glaze in a bowl or on a plate, but only a single glaze on the outside of any vertical surface! Glazes are a chemical reaction under heat. We don’t know the exact chemicals in the Mayco glazes we use. I can guess by the way they interact with each other, however, on the whole, you need to test every idea you have, and not run the test on a vertical surface! Simply put, glaze is a layer of glass like substance that bonds with the clay underneath. Clay is made of silica, alumina and water. Glaze is made of mostly silica. Silica has a melting point of 1700°C and we fire to 1240°C. The silica requires a “flux” to help it melt at the lower temperature. Fluxes can be all sorts of chemicals – a common one is calcium – calcium has a melting point of 2500°C, however, together they both melt at a much lower temperature! Colourants are metal oxides like cobalt (blue), chrome (green through black), copper (green, blue, even red!), manganese (black, purple and pink) iron (red brown), etc. Different chemicals in the glaze can have dramatic effects. for example, barium carbonate (which we don’t use) turns manganese bright pink! Other elements can turn manganese dioxide brown, blue, purple and reddish brown. Manganese dioxide is a flux in and of itself as well. So, glazes that get their black and purple colours, often interact with other glazes and RUN! Our mirror black is a good example – it mixes really well with many glazes because it fluxes them – causes them to melt faster. It will also bring out many beautiful colours in the glazes because it’s black colouring most definitely comes from manganese dioxide! Glaze chemistry is a whole subject on it’s own! We use commercial Mayco glazes on purpose – for their huge range of colour possibilities, stability, cool interactions, artistic freedom with the ability to easily brush the glazes on and ease of use. We currently have almost 50 glazes on hand! A major project is to test the interactions of all glazes with each other. That is 2,500 test tiles!!!! I’m going to make the wall behind the wheels the feature wall of pretty colours! Step Seven – Glaze (Gloss or sometimes called “Glost”) Firing Most of the time this is the final stage of making your creation (but not always!) The glaze kiln goes to 1240°C. This is called cone 6, or midrange. It is the low end of stoneware temperatures. Stoneware clays and glazes are typically fired at cone 8 – 10, that is 1260 – 1290°C. The energy requirement to go from 1240°C to 1280°C is almost a 30% more! Our clay is formulated to vitrify (mature, turn “glass-like”) at 1240°, as are our glazes. A glaze kiln take around 12 hours to reach temperature and two to three days to cool down. Sometimes a third firing process is required – this is for decoration that is added to work after the glaze firing. For example – adding precious metals and lustres. this firing temperature is usually around 600 – 800°C depending upon the techniques being used. There are many students interested in gold and silver trims – we will be doing this third type of firing soon! After firing your work will be in the student finished work shelves. Remember to pay for it before you head out the door! There is a small extra charge for using porcelain clay (it’s more than twice the price of regular clay), and for any third firing process! Once your work has been fired it can not turn back into clay for millennia – so don’t fire it if you don’t like it! Put it in the bucket for recycling. https://firebirdstudios.com.au/the-process-of-making-pottery/The form of the jug has been in use for many centuries.Stoneware jug. Two tone brown glaze with pierced lip behind spout. Spout chipped.None.flagstaff hill, warrnambool, shipwrecked-coast, flagstaff-hill, flagstaff-hill-maritime-museum, maritime-museum, shipwreck-coast, flagstaff-hill-maritime-village, jug, ceramic jug -
 Federation University Historical Collection
Federation University Historical CollectionBook, The Pyreness Shire, Avoca Shire Heritage Study 1864-1994, Volume 1, 1995
The Pyreness Shire, Avoca Shire Heritage Study 1864-1994, Volume 11) 21008.1 - Volume 1 - Pale blue bound book of 47 pages - Environmental History 2) 21008.2 - Volume 3 - Pale blue bound book - Geographical Locations of Individual Sites, alphabetically by Road Namewendy jacobs, karen twigg, nigel lewis/richard aitken pty ltd, shire of avoca, avoca heritage study, national estate committee (victoria), national estate grants program, victorian goldfields, pyrenees, moonambel, natte yallock, rathscar, barkly, redbank, crowlands, landsborough, the pyrenees shire, lexton shire, exploration and pastoralism, gold, water, farming, wine and fruit, towns and settlements, living in community, road and rail, extractive industries, conclusion, ballarat, major mitchell, djadja wurrung aboriginal group, djab wurrung aboriginal group, avoca, lamplough, chinese camp, alluvial mining, ironbark mine, upper homebush, homebush deep lead mine, avoca and district historical society, deep leads, quartz mining, percydale, hog's reef mine, avoca, dredging, hunter's home, moonambel c. 1890, mrs ellen allan, lamplough, the 1865 land act, flour milling, flour mill, moonambel. c. 1880, harkins farm, bung bong c. 1900, dairying, viticulture, navarre, schools, churches, cemetaries, wars, hotels, halls, sports, horse racing, country fire authority, maryborough-avoca railway, cobb and co -
 Federation University Historical Collection
Federation University Historical CollectionPhotograph, Clare Gervasoni, Memorial to Francis Davis in the grounds of Federation University, SMB Campus, 2019, 20/01/2019
... . Major K.D. Norman C. of E. A.I.F. officiated. The grave..." was sounded at the graveside, and the Rev. Major K.D. Norman C. of E ...Francis Gordon Davis was born in Ballarat on 09 August 1899. He is the only former student of the Ballarat Junior Technical School who was killed on service during World War One. Davis enlisted into the Australian Flying Corps, Laverton, on 12 April 1918 at which time he was 18 and 8 months and served as a second class Air Mechanic. His service number was 3310. He died accidentally from shock resulting from an accident resulting from skidding a Leyland Motor Lorry at Leighterton, Tetbury, Gloucester, England on 28 January 1919 and is buried in Grave 6 in the Soldiers Corner of the Leighterton Cemetery. Francis Davis was accorded a full military funeral, firing party, bugler and pallbearers. The coffin was draped in the Union Jack and surmounted in several beautiful wreathes sent from his brother 2/A.M. E.H. Davis (A.F.C Leighterton), officer of the A.F.C. Leighterton, Gloucester, Cadets of A.F.C. and many other personal friends of the deceased. The "Last Post" was sounded at the graveside, and the Rev. Major K.D. Norman C. of E. A.I.F. officiated. The grave was to be turfed and an oak cross erected by the A.I.F. London. Administrative Headquarters A.I.F. London were represented at the funeral. (http://bih/index.php/Francis_G._Davis) In June 1922 Alfred Davis, the father of Francis Davis, planted a tree in the grounds of the Ballarat Junior Technical School in honour of hos son. It was the first tree of six planted in the grounds of the Ballarat School of Mines on Arbor Day 1922. Speaking of the planting of the tree by Mr Davis the Chief Secretary (Mr M. Baird M.L.A.), said he trusted the memory would ever remain green at the school. Had he and others not given their lives nothing that we could have done to-day could have retrieved the time. Australians had indeed done splendidly, but they should take a wider outlook than Australia, and reading the history of the Genoa Conference he had been struck by what had been done ... We should honor such men as he in whose memory that tree was planted, and the schools that sent them out to fight for us. He hoped the empire would always be able to produce such men, so that the Empire would always be able to lead the World's struggle for the benefit of humanity. The last post was then sounded by Mr. H. Green. ... (Ballarat Courier, 19 June 1922)A number of photographs of a tree and marble plaque in the grounds of the Ballarat School of Mines. It was a memorial to Francis Davis, a former student of the Ballarat Junior Technical School, who died on active service during World War One.ballarat school of mines, ballarat junior secondary school, world war one, memorial, marble plaque, marble memorial, tree, memorial tree, davis, francis davis, centenary
